Disaster Management
Future Pandemic Preparedness And Emergency Response
- 21 Nov 2024
- 22 min read
For Prelims: NITI Aayog, Future Pandemic Preparedness, One Health approach, Public Health Emergency Management Act, Genomic Tools, Vaccines, Zoonotic Diseases, WHO, Public Health Emergencies of International Concern (PHEICs), Covid-19, Antibiotic Resistance, Biosafety and Biosecurity, Bioterrorism
For Mains: India’s Future Pandemic Preparedness, Viability of Public Health Emergency Management Act.
Context
Recently, the Expert Group constituted by NITI Aayog on Future Pandemic Preparedness submitted its final report titled "Future Pandemic Preparedness and Emergency Response — A Framework for Action."
- The report highlights lessons learned from the Covid-19 pandemic, global best practices, and recommendations for strengthening India's public health crisis management.
What was the Need to set up the Expert Panel on Pandemic Preparedness?
- Lessons From India's Response to Covid-19:
- As India recovers from Covid-19, the worst health crisis in recent history, it is essential to draw lessons and build robust preparedness frameworks for future pandemics.
- The Global Health Landscape and Future Risks:
- Globally, science-based measures like epidemiologic surveillance, genomic tools, diagnostics, and vaccines were crucial in tackling the virus.
- India adopted a whole-of-government approach, overcoming challenges that offer critical learnings for future preparedness within a 100-day mission framework.
- Future pandemics are inevitable due to ecological changes, zoonotic threats, and evolving human-animal dynamics.
- The World Health Organization (WHO) warns that 75% of future health threats may be zoonotic.
- Over the past two decades, WHO declared seven Public Health Emergencies of International Concern (PHEICs), including H1N1, Ebola, Zika, and Covid-19, highlighting the unpredictability and complexity of health crises.
- India’s Preparedness Strategy and One Health Mission:
- India’s One Health (OH) Mission integrates human, animal, and environmental health through effective surveillance, outbreak response, and research.
- However, threats like bioterrorism, resistant pathogens, and climate change impact demand strategies beyond the OH approach, involving national security and global collaboration.
- India’s One Health (OH) Mission integrates human, animal, and environmental health through effective surveillance, outbreak response, and research.
- Global Initiatives and Future Preparedness Frameworks:
- Globally, initiatives like WHO's identification of high-risk pathogens, the revised International Health Regulations, and the Pandemic Accord negotiations emphasize preparedness.
- Countries must align with frameworks like Preparedness and Resilience for Emerging Threats (PRET) to enhance epidemiological capacity and meet obligations under international health regulations.
- Globally, initiatives like WHO's identification of high-risk pathogens, the revised International Health Regulations, and the Pandemic Accord negotiations emphasize preparedness.
Past Epidemics in Last Two Decades
|
Disease |
Key Characteristics |
Lessons Learned |
|
Highly infectious, respiratory spread, global outbreak |
Need for international regulations, rapid diagnostics, core capacities at airports |
|
|
Avian Flu (H5N1) (Since 2005) |
Zoonotic disease, primarily affects poultry, occasional human cases |
Effective surveillance, coordinated response, and a standing committee on zoonosis |
|
H1N1 Pandemic (2009) |
Respiratory spread, declared as PHEIC |
Importance of International Health Regulations (IHR) (2005), core capacities, public health measures, and coordinated surveillance |
|
Ebola Outbreaks (2014-2016, 2018-2021) |
Highly infectious, primarily in Africa, international spread |
Effective screening, surveillance, contact tracing, and public health measures |
|
MERS-CoV (since 2012) |
Zoonotic disease, respiratory spread, outbreaks in Middle Eastern countries |
Challenges in preventing zoonotic diseases, especially those with respiratory transmission |
|
Zika Virus Disease (since 1950s) |
Vector-borne disease, asymptomatic in many cases |
Importance of vector surveillance and control, multi-sectoral collaboration |
What Lessons and Challenges Emerged from COVID-19?
- Governance:
- The Covid-19 response highlighted effective collaboration between government levels and agencies, aided by Empowered Groups and National Task Forces that enabled rapid decision-making.
- Science-based evidence supported informed actions as understanding of the virus evolved.
- However, there was a need for clearer roles and better coordination between agencies. Risk communication was a gap, as there wasn’t an efficient system for two-way data sharing.
- Additionally, a rapid response plan and clearer delegation of authority were essential to enable swift, time-bound actions without delays from hierarchical processes.
- The Covid-19 response highlighted effective collaboration between government levels and agencies, aided by Empowered Groups and National Task Forces that enabled rapid decision-making.
- Legislation:
- The National Disaster Management Act (NDMA), 2005 allowed quick response and public health measures during Covid-19, but there's a need for a dedicated Public Health Act.
- NDMA provisions don’t fully address the unique needs of public health and clinical management in emergencies, and the older Epidemic Act, 1897 lacks the scope to handle modern pandemic requirements effectively.
- Surveillance and Data Management:
- Covid-19, caused by a new zoonotic virus, needed a One Health approach, but data collection was difficult due to fragmented information from various sources.
- There was no unified system to analyze this data for effective modeling and forecasting.
- A single National Data Portal is recommended to aggregate and analyze data from all sources for trend identification and outbreak prediction.
- New digital tools (Aarogya Setu, CoWIN) aided contact tracing and vaccination but needed better integration with hospital networks.
- The Indian SARS-CoV-2 Genomics Consortium (INSACOG) genomic surveillance network identified new strains but lacked strong links with state and private labs.
- Expanding INSACOG and connecting it to clinical data for early warnings is crucial.
- Covid-19, caused by a new zoonotic virus, needed a One Health approach, but data collection was difficult due to fragmented information from various sources.
- Research and Development, Translation, and Product Development:
- Collaboration between the public and private sectors was key in managing the pandemic.
- Industries excelled in research and manufacturing, but better links with institutions like the Indian Council of Medical Research (ICMR) and stronger supply chains are needed for large-scale, cost-effective production.
- Collaboration between the public and private sectors was key in managing the pandemic.
- Regulatory Reforms:
- During Covid-19, a rapid regulatory framework was created, but it wasn’t fully prepared for quick emergency approvals.
- Clearer guidelines and alignment with global regulatory standards are essential, especially for new technologies.
- Delays also occurred due to a lack of harmonized international guidelines, even for products approved by other countries.
- A strong, globally recognized network of clinical trial sites is needed to support trials of internationally developed products.
What are Future Pandemic Threats and Strategies for Preparedness?
- Rising Risk of Emerging Infectious Diseases: The risk of diseases caused by known and unknown pathogens, especially zoonotic ones (from animals, birds, wildlife), has increased due to:
- Intensified international travel, trade, and livestock husbandry.
- Growing human population density.
- Changing interactions between humans and wild animals.
- Ecological changes such as climate change and global warming, amplify disease risks.
- WHO Global Priority Pathogen List: The WHO is updating a global list of priority pathogens to guide research and development (R&D) investments, focusing on vaccines, tests, and treatments. The current list includes:
- Covid-19, Ebola, Crimean-Congo haemorrhagic fever, MERS, SARS, Nipah, and others, alongside Disease ‘X’.
- A separate list for antibiotic-resistant bacterial pathogens includes 15 families, categorized by priority for R&D and public health measures.
- Collaborative Surveillance:
- Timely detection of emerging pathogens and assessing their public health impact is crucial.
- For better planning, surveillance should track disease spread, severity, and outcomes across communities and health facilities.
- Preparedness Strategy:
- Cross-sectoral and Cross-border Collaboration: Enhanced coordination between public health authorities, disaster management agencies, and other sectors is crucial for managing pandemic threats effectively.
- Risk Assessment and Community Engagement: Strategies should be in place to assess potential risks, address misconceptions or rumors during outbreaks, and ensure accurate information dissemination and community cooperation.
- Resource Availability: Ensuring sufficient funds and resources are available to support effective pandemic response efforts.
- One Health Approach: Developing a multi-hazard plan and strategies for coordinated surveillance and response to zoonotic and emerging infectious diseases.
What are the Recommendations of the Report?
- Governance:
- Empowered Groups for Rapid Response: It is proposed that a Standing Empowered Group of Secretaries (EGoS) be created for Pandemic Preparedness and Emergency Response (PPER), chaired by the Cabinet Secretary and co-chaired by health officials.
- This group would ensure effective coordination, approvals, and monitoring of pandemic preparedness during peacetime.
- Institutionalizing Governance Structures: Existing governance frameworks should be institutionalized, with operational manuals/SOPs and regular drills (e.g., war-room operations) for preparedness.
- Empowered Groups for Rapid Response: It is proposed that a Standing Empowered Group of Secretaries (EGoS) be created for Pandemic Preparedness and Emergency Response (PPER), chaired by the Cabinet Secretary and co-chaired by health officials.
- Legislation:
- Need for Specialized Public Health Legislation: Existing laws like the Epidemic Diseases Act (1897) and the National Disaster Management Act (2005) are not sufficient to address modern health emergencies.
- A new Public Health Emergency Management Act (PHEMA) is proposed to address issues beyond epidemics, such as non-communicable diseases, disasters, and bioterrorism.
- Global Examples: Other countries, like the USA, have comprehensive laws (e.g., Public Health Service Act) that provide a legal framework for managing public health emergencies, which India should adopt.
- Need for Specialized Public Health Legislation: Existing laws like the Epidemic Diseases Act (1897) and the National Disaster Management Act (2005) are not sufficient to address modern health emergencies.
- Finance and Management:
- Pandemic Preparedness Fund: A special Pandemic Preparedness and Emergency Response Fund is proposed to ensure readiness for future public health crises. This fund should be established in advance, allowing for rapid action and a 100-day response time.
- Data Management (Generation/Sharing/Analysis):
- Unified Data Platform: Build a unified platform to integrate all data portals, enabling seamless data flow and analysis for quick decision-making.
- Unified System: Create a Unified Data Management System, supported by a National Analytical Cell to interpret and utilize data effectively.
- Data Communication:
- Timely Communication: Establish an empowered Data Analysis and Reporting Unit at NCDC to ensure correct, timely data sharing and public communication.
- Delegated Powers: Develop a manual of pre-approved delegated powers to facilitate smooth data communication.
- Surveillance:
- Strengthen Surveillance Network: Enhance the current surveillance system, ensuring seamless coordination across center, state, and district levels for real-time alerts.
- Integrated Systems: Develop a One Health approach to connect surveillance systems across public/private sectors, including biosecurity at borders and ports.
- Genomic Surveillance: Strengthen genome sequencing and wildlife surveillance to monitor pathogen mutations and track trans-boundary pathogen movement.
- Emergency Operations Center (EOC): Establish a network of EOCs at district levels, integrating data flow for prompt decision-making.
- Bats and Animal Surveillance: Focus on bat species as reservoirs for zoonotic diseases, incorporating the One Health approach.
- Forecasting & Modelling:
- Build Predictive Models: Establish a robust network for epidemiology forecasting and modeling, using reliable Indian data to create predictive models on transmission dynamics.
- AI and Emerging Technologies: Leverage AI and new technologies in predictive modelling efforts.
- Epidemiology Forecasting Network: Create centres of excellence for epidemiological forecasting, including mathematical modelling and partnerships across public, private, and academic sectors.
- Data Integration: Use surveillance data (clinical, genomic, sewage) for modelling trends and predicting outbreaks, with active involvement from institutions like ICMR and IITs.
- National Biosafety & Biosecurity Network:
- Integrated Approach: Develop a National Biosecurity and Biosafety Network to address pathogens affecting humans, animals, and plants, adopting a One Health approach.
- Research and Development: Establish biosafety containment facilities (BSL-2, BSL-3, BSL-4) and a network of diagnostic labs for quick response to emerging threats.
- Research & Innovation:
- Focus on Zoonotic Epidemics: Research and innovation, especially in vaccine and diagnostic development, are crucial for combating pandemics.
- High-Risk Innovation Fund: Establish a dedicated fund to support R&D for diagnostics, therapeutics, vaccines, and cutting-edge technologies, with emphasis on priority pathogens.
- Diagnostics and Therapeutics:
- Diagnostic Development: Develop indigenous diagnostic kits, addressing challenges like dependency on imported reagents.
- Therapeutic Development: Launch a National Mission on Therapeutics to focus on drug development for priority pathogens, with an emphasis on both repurposing approved drugs and identifying novel compounds.
- Public-Private Partnerships (PPP): Involve private sectors and startups in the R&D process, ensuring rapid response and development of necessary drugs and vaccines.
- Regulatory Reforms:
- Accelerated Approvals: Develop a harmonized regulatory system for faster approval of vaccines, diagnostics, and therapeutics, along with an independent regulatory authority for rapid decision-making.
- Strengthen Clinical Trials: Establish a robust clinical trial network and adaptive methodologies to speed up drug and vaccine development.
- Resilient Supply Chains:
- Innovation & Vaccine Science:
- Establish an Innovation and Vaccine Science & Development Institute to focus on research, development, and scaling up of vaccines for emerging pathogens, integrating human and animal health perspectives.
- Innovation & Vaccine Science:
- Community Involvement and Engagement with the Private Sector:
- Risk Communication & Community Engagement (RCCE): Effective planning and control strategies during pandemics require active community engagement and risk communication.
- Private Sector Role: Private labs and hospitals should be involved in all phases of a pandemic, from surveillance to post-pandemic monitoring.
- Pre-Pandemic: Establish partnerships with private sector hospitals and labs for surveillance and planning.
- Pandemic: Involve the private sector in testing, genome sequencing, and providing healthcare support.
- Post-Pandemic: Monitor mutations, variants, and long-term health impacts (e.g., long Covid).
- Communication:
- Risk Communication Unit: A dedicated unit at National Centre for Disease Control (NCDC), led by a senior officer, should handle updates and ensure effective communication with all stakeholders, including the community.
- Infodemic Management: Develop strategies for managing misinformation and educating the public through partnerships with institutions like United Nations Children's Fund (UNICEF) and behavioral science experts.
- Collaborations and Partnerships:
- Collaboration for Success: Partnerships between academia, industry, government, and international organizations were crucial for scientific breakthroughs during the pandemic.
- Pre-Agreed Protocols: Establish Memorandums of Understanding (MoUs), protocols, and agreements for data, knowledge sharing, and collaborative funding to expedite responses in future health emergencies.
- International Partnerships: Strengthen international collaborations for information sharing, technology transfer, and ensuring global regulatory approvals for vaccines and therapeutics.
- Institutionalizing Collaboration: Focus on collaborative learning during non-crisis periods to build systems that are ready for future pandemics.
- Collaboration for Success: Partnerships between academia, industry, government, and international organizations were crucial for scientific breakthroughs during the pandemic.
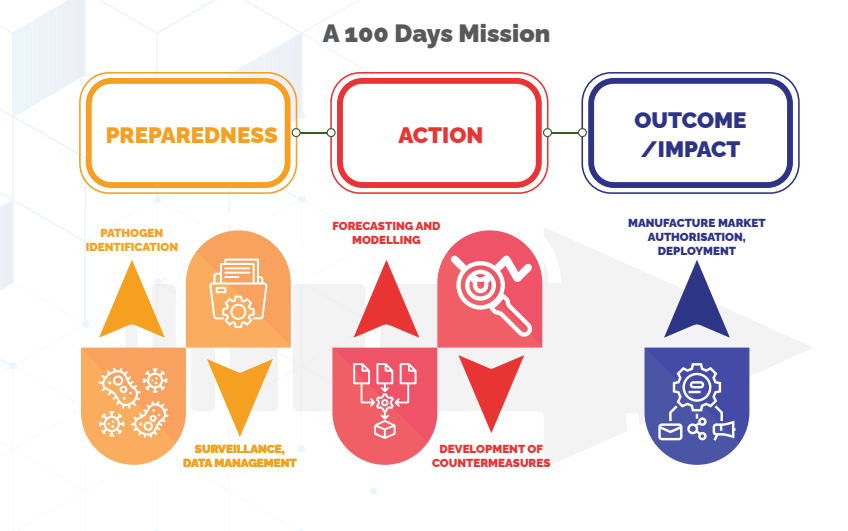
What is the 100-Day Mission Framework for Preparing for Future Pandemics?
- Preparedness:
- Public Health Emergency Management Act: Establish a legal framework for pandemic preparedness.
- EGoS on Pandemic Preparedness and Response: Form empowered groups for strategic planning and response.
- High-Risk Innovation Fund: Allocate funds for R&D focused on pandemic preparedness and response.
- Robust Surveillance System: Develop a connected network integrating genomic, epidemiological, clinical, and hospital data.
- Unified Data Management: Centralized system for data collection and analysis.
- Forecasting and Modelling: Predict pandemic trends and manage resources effectively.
- Priority Pathogen Research: Focus on key pathogens for early intervention.
- Strain Characterization: Maintain a repository of well-characterized and sequenced strains.
- Diagnostics and Vaccines: Develop prototypes for diagnostics and vaccines for priority pathogens.
- Pre-Approved SOPs: Set standard procedures for rapid regulatory approval, data communication, and international agreements.
- 100 Days Response:
- Infection and Pathogen Tracking: Quickly identify pathogens for targeted response.
- Sensitive Diagnostics: Develop diagnostics and scale manufacturing.
- Vaccine Development: Develop vaccines tailored to specific pathogens.
- Therapeutics/Drugs: Develop treatments for emerging diseases.
- Early Forecasting: Predict disease trends and implement protocols in hot spots.
- Rapid Response Teams: Deploy teams from day one.
- Continuous Data Analysis: Monitor and analyze data to guide research and health systems.
- Strain Sharing: Share characterized strains and samples across organizations.
- Accelerated Regulatory Approvals: Harmonize regulatory processes for faster approval of countermeasures.
- Output and Impact:
- Mass Deployment: Ensure countermeasures are available for large-scale public health response.
- Continuous Surveillance: Ongoing collection of epidemiological, clinical, and genomic data for managing disease in hotspots.
- Rapid Response: Immediate action by response teams following SOPs.
- Regular Communication: Continuous risk communication and community engagement.
- Efficient Disease Management: Prevent, treat, and manage diseases with minimal infections.
UPSC Civil Services Examination, Previous Year Questions (PYQs)
Prelims:
Q1. In the context of vaccines manufactured to prevent Covid-19 pandemic, consider the following statements: (2022)
- The Serum Institute of India produced Covid-19 vaccine named Covishield using mRNA platform.
- Sputnik V vaccine is manufactured using vector-based platform.
- COVAXIN is an inactivated pathogen-based vaccine.
Which of the statements given above are correct?
(a) 1 and 2 only
(b) 2 and 3 only
(c) 1 and 3 only
(d) 1, 2 and 3
Ans: (b)
Mains:
Q1. Covid-19 pandemic has caused unprecedented devastation worldwide. However, technological advancements are being availed readily to win over the crisis. Give an account of how technology was sought to aid management of the pandemic. (2020)