Important Facts For Prelims
India’s CAR T-Cell Therapy
- 19 Mar 2025
- 4 min read
Why in News?
The clinical trial results of India’s first Chimeric Antigen Receptor (CAR) T-Cell Therapy, published in The Lancet Haematology, report a 73% response rate in leukemia and lymphoma patients.
What are the Key Findings of India’s CAR T-Cell Therapy Clinical Trial?
- High Success Rate: The trial involved patients with relapsed or refractory B-cell cancers (Leukemia (cancer affecting bone marrow and blood) and Lymphoma (cancer of the lymphatic system)), who often have limited treatment options.
- Among the patients analyzed, 73% showed a positive response to the therapy, offering new hope for such cases.
- Comparable to Global Therapies: India's CAR T-cell therapy matches global effectiveness but is 20 times cheaper, costing Rs 25 lakh compared to Rs 3-4 crore internationally, where total expenses can exceed Rs 8 crore.
- Side Effects Observed: The clinical trials of India’s CAR T-cell therapy reported manageable side effects, with patients experiencing neutropenia (low white blood cells), thrombocytopenia (low platelets), and developing anemia (low red blood cells).
- Some patients showed cytokine release syndrome (CRS), causing fever and inflammation.
- Two treatment-related deaths were reported, but overall, the safety profile was considered manageable.
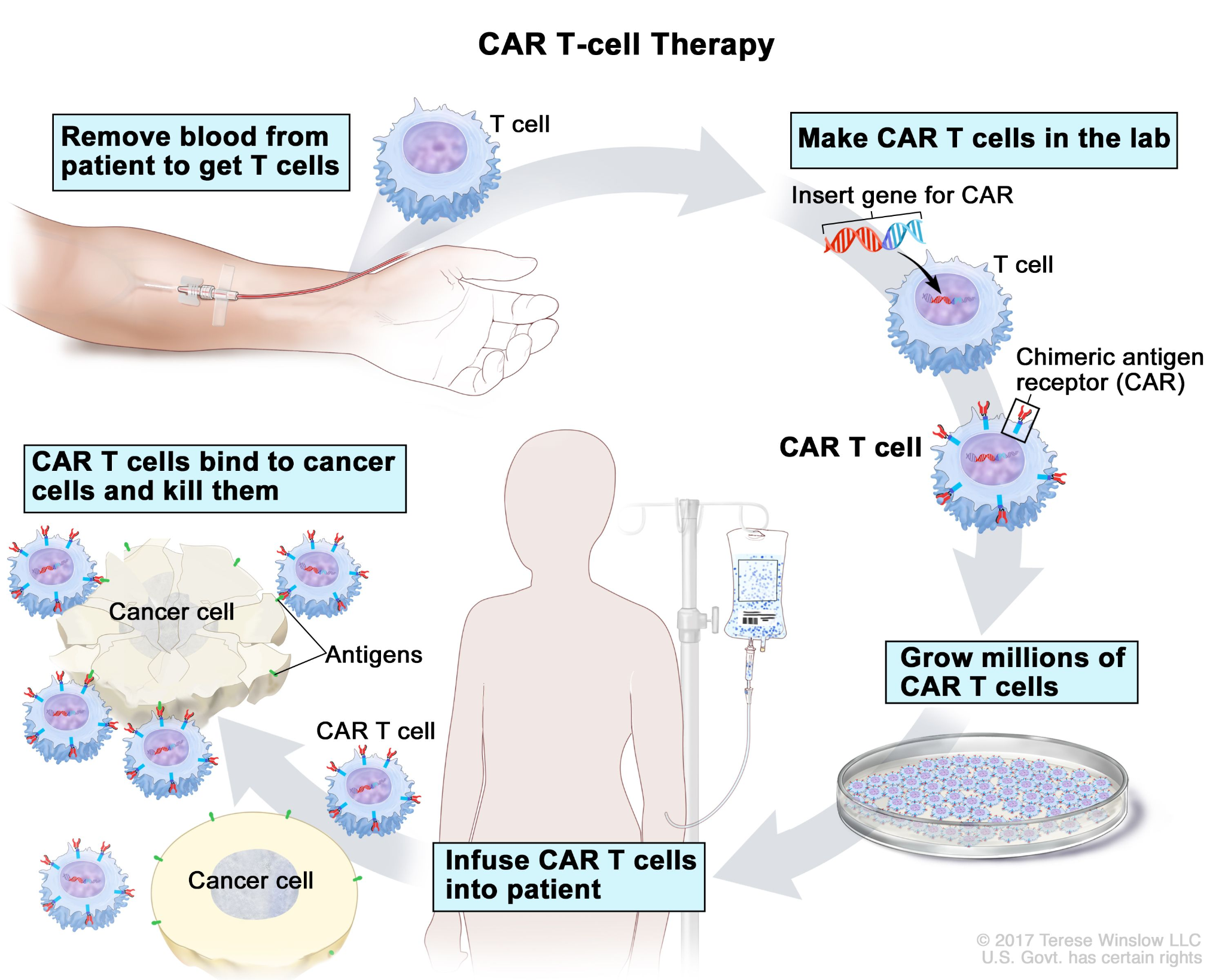
What is CAR T-Cell Therapy?
- About: CAR T-cell therapy is an advanced cancer treatment that modifies a patient’s T-cells (a type of immune cell) to fight cancer more effectively.
- Working: A patient's T-cells are extracted from their blood and genetically modified (to recognize and attack cancer cells).
- These modified cells, known as Chimeric Antigen Receptor (CAR) T-cells, are multiplied and reintroduced into the patient to target B-cells and prevent relapses.
- Importance: When B-cell tumors relapse or become refractory (return after treatment or do not respond to initial therapy), treatment options are limited, often leading to patient death.
- Uncontrolled B-cell growth causes severe complications due to their role in antibody production.
- India's CAR T-cell therapy provides an additional, patient-specific treatment option, as the modified T-cells remain in the body, offering long-term immunity against cancer recurrence.
- It is a patient-specific treatment, making it highly precise compared to traditional chemotherapy.
- NexCAR19: In 2023, NexCAR19 became India’s first approved indigenous CAR-T cell therapy, developed through a collaboration between IIT Bombay, Tata Memorial Centre, and ImmunoACT (a company incubated at IIT Bombay).
- As the world's most affordable CAR-T therapy, it positions India on the global map for advanced cell and gene therapy.
- Implications: Researchers are exploring CAR T-Cell Therapy applications and combination with immunotherapies, paving the way for broader adoption of gene-modified cell treatments in India.
UPSC Civil Services Examination, Previous Year Question (PYQ)
Prelims
Q. Which one of the following statements best describes the role of B cells and T cells in the human body? (2022)
(a) They protect the environmental allergens. body
(b) They alleviate the body’s pain and inflammation.
(c) They act as immunosuppressants in the body.
(d) They protect the body from diseases caused by pathogens.
Ans: (d)