Agriculture
Haber-Bosch Process and Production of Fertilizers
- 17 Oct 2024
- 9 min read
For Prelims: Haber-Bosch process, Nitrogen, Ammonia, Lightning, Azotobacter and Rhizobia, Volcanic eruptions, Acid rain, organic farming, biofertilizers.
For Mains: Importance of the Haber-Bosch process, Implications of Using Fertilizers, Nitrogen cycle.
Why in News?
Through the Haber-Bosch process, a hundred million tonnes of nitrogen are extracted from the atmosphere and transformed into fertiliser, resulting in the addition of 165 million tonnes of reactive nitrogen to the soil.
- In comparison, natural biological processes generate an estimated 100-140 million tonnes of reactive nitrogen annually.
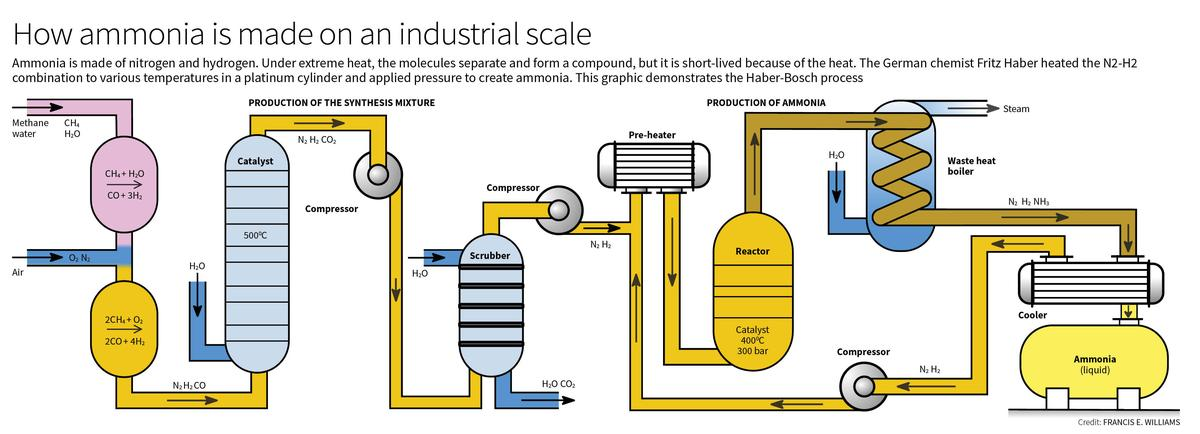
What is the Haber-Bosch Process?
- About:
- The Haber-Bosch process is an industrial method for synthesizing ammonia by combining nitrogen from the air with hydrogen, significantly contributing to fertiliser production.
- Process:
- Experimental Setup:
- The reaction occurs in a steel chamber at a pressure of 200 atm, allowing the nitrogen-hydrogen mixture to circulate effectively.
- A specially designed valve withstands high pressure while allowing the N₂-H₂ mixture to flow through.
- Haber implemented a system to transfer heat from the outgoing hot gases to the incoming cooler gases, optimizing energy efficiency.
- Catalyst Development:
- Haber initially experimented with various materials looking for suitable filament materials as Catalyst to speed up reaction.
- Among the tested materials was osmium, which, when placed in the pressure chamber with the N₂-H₂ mixture, successfully cracked the nitrogen triple bond, allowing for ammonia production.
- Uranium was another effective catalyst but both osmium and uranium were too expensive for large-scale applications.
- The search for a more cost-effective catalyst led to the identification of specific iron oxides as viable options.
- Experimental Setup:
- Applications:
- Manufacturing: As a refrigerant in industrial refrigeration systems and air conditioning.
- Household: An ingredient in household cleaning products, including glass and surface cleaners.
- Automotive fuel: An internal combustion engine powered by ammonia is being explored as an alternative propulsion technology.
- Chemicals: A precursor for various chemicals, including nitric acid and explosives.
- Key Milestones:
- In 1913, the German chemical company opened its first ammonia factory, marking a significant milestone in the production of fertilizers.
- Fritz Haber, a German chemist, won the Nobel Prize in Chemistry in 1919 for his work on ammonia synthesis.
What is the Nitrogen Cycle?
- About:
- Plants obtain reactive nitrogen from the soil by absorbing nitrogen-based minerals like ammonium (NH4+) and nitrate (NO3–), dissolved in water.
- Humans and animals rely on plants for nine essential nitrogen-rich amino acids, as nitrogen makes up about 2.6% of the human body.
- After being ingested, nitrogen returns to the soil through excreta and decomposition of dead organisms, but some nitrogen escapes back into the atmosphere as molecular nitrogen, leaving the cycle incomplete.
- Natural Availability of Nitrogen:
- Lightning: Lightning bolts possess enough energy to break the N2 bond, combining nitrogen with oxygen to form nitrogen oxides (NO and NO2).
- These oxides mix with water vapour, forming nitric and nitrous acids, which fall as acid rain, providing reactive nitrogen to the soil.
- Biological Fixation: Some bacteria, like Azotobacter and Rhizobia, can convert atmospheric nitrogen into reactive nitrogen.
- These bacteria often have symbiotic relationships with plants such as legumes or aquatic ferns like Azolla, which enhance nitrogen availability in the soil, making them valuable for agriculture.
- Process of Nitrogen Replenishment:
- While legumes can fix nitrogen naturally, most staple crops like rice, wheat, corn, potatoes, cassava, bananas, and other fruits and vegetables depend on soil nitrogen for growth.
- As human populations grow, the depletion of nitrogen in agricultural soils accelerates, requiring the use of fertilizers to restore soil fertility.
- Historical Fertilization Methods:
- Farmers historically cultivated legumes to naturally replenish nitrogen in the soil or applied ammonia-based fertilizers to increase crop yields.
- They also utilized ammonium-rich minerals from volcanic eruptions and naturally occurring nitrates found in caves and rocks to enhance soil fertility.
- Lightning: Lightning bolts possess enough energy to break the N2 bond, combining nitrogen with oxygen to form nitrogen oxides (NO and NO2).
What is the Impact of Industrial Production of Fertilizers?
- Pros:
- The Haber-Bosch process enabled the mass production of synthetic fertilizers, significantly boosting global food supply during the 20th century, contributing to increased life expectancy.
- An estimated one-third of the world's population relies on food produced using nitrogen fertilizers.
- Without the industrial production of ammonia from nitrogen and hydrogen, it would have been impossible to meet the growing global demand for food.
- Cons:
- Synthetic nitrogen fertilizers, although critical for food production, have adverse environmental impacts.
- Excess nitrogen application leads to plant over-nourishment, boosting bacterial activity and accelerating nitrogen release into the atmosphere.
- This contributes to environmental degradation, including acid rain, land corrosion, and surface water deoxygenation through runoff, causing excessive weed growth in water bodies.
Way Forward
- Promote Sustainable Fertilizer Use: Encourage the adoption of precision agriculture and controlled-release fertilizers to reduce nitrogen waste, minimize environmental damage, and enhance the efficiency of fertilizer usage in farming.
- Invest in Alternative Technologies: Develop and promote eco-friendly alternatives to synthetic fertilizers, such as organic farming practices, nitrogen-fixing crops, and biofertilizers, to mitigate the environmental impacts of chemical fertilizers.
- Strengthen Policy Frameworks: Governments should implement regulations to control fertilizer overuse and incentivize sustainable farming practices, ensuring food security while protecting ecosystems and public health.
- Enhance Global Cooperation: Foster international collaboration to address food distribution disparities, improve access to agricultural innovations, and support capacity-building initiatives for regions facing food insecurity, ensuring equitable solutions to global food challenges.
|
Drishti Mains Question: Critically examine the impact of synthetic fertilizers on agriculture and the environment. Discuss sustainable alternatives to mitigate these challenges. |
UPSC Civil Services Examination, Previous Year Question (PYQ)
Prelims:
Q. With reference to chemical fertilizers in India, consider the following statements: (2020)
- At present, the retail price of chemical fertilizers is market-driven and not administered by the Government.
- Ammonia, which is an input of urea, is produced from natural gas.
- Sulphur, which is a raw material for phosphoric acid fertilizer, is a by-product of oil refineries.
Which of the statements given above is/are correct?
(a) 1 only
(b) 2 and 3 only
(c) 2 only
(d) 1, 2 and 3
Ans: (b)
Mains:
Q. Sikkim is the first ‘Organic State’ in India. What are the ecological and economical benefits of Organic State? (2018)