Biodiversity & Environment
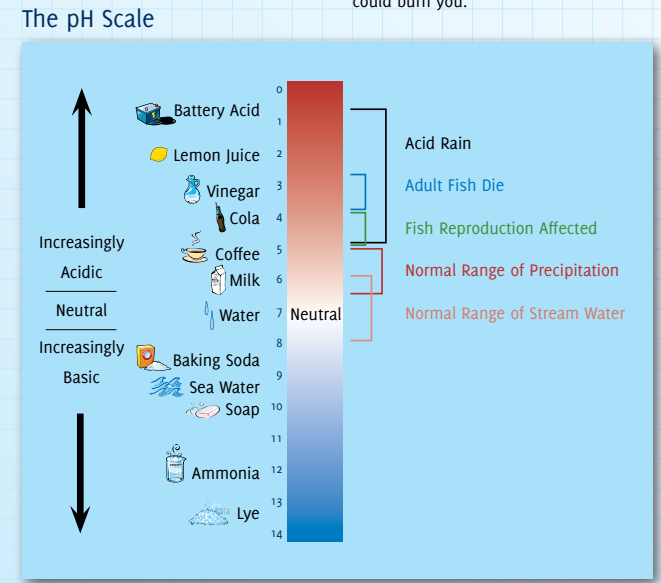
Acid Rain
- 01 Feb 2024
- 11 min read
For Prelims: Fossil Fuels, Acid Rain, Air Pollution, flue-gas desulphurisation, Acid Deposition Monitoring Network in East Asia (EANET).
For Mains: Acid Rain, Environmental pollution and degradation.
Why in News?
Acid Rain is a complex environmental issue with multiple causes and widespread consequences, and it has its origins in Fossil Fuels.
What is Acid Rain?
- About:
- Acid rain or acid deposition is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms.
- This can include rain, snow, fog, hail or even dust that is acidic.
- Formation of Acid Rain:
- When SO2 (Sulphur Dioxide) and NOx (Nitrogen Oxide) combine with water and oxygen in the atmosphere, they form sulfuric acid (H2SO4) and nitric acid (HNO3), respectively.
- These acids then dissolve in water droplets, leading to the creation of acid rain, snow, or fog.
- The typical pH (Potential of Hydrogen) of acid rain is around 4.2-4.4, making it more acidic than normal rain (which has a pH of around 5.6).
- Causes of Acid Rain:
- Fossil Fuel Combustion: Burning Fossil Fuels, particularly those containing sulfur, release sulfur dioxide (SO2) and, at higher temperatures, nitrogen oxides (NOx).
- Fossil fuel combustion is prevalent in vehicles such as automobiles, and is a primary source of environmental pollutants.
- The combustion of coal in power plants and industrial processes also releases these substances.
- Natural Sources: Volcanic Eruptions and Lightning also contribute to the presence of sulfur dioxide and nitrogen oxides in the atmosphere.
- Air Pollution: In the atmosphere, the pollutants SO2 and NOx undergo chemical reactions, forming sulfuric and nitric acids.
- When combined with water vapor, they create acid rain during precipitation.
- Fossil Fuel Combustion: Burning Fossil Fuels, particularly those containing sulfur, release sulfur dioxide (SO2) and, at higher temperatures, nitrogen oxides (NOx).
- Forms of Acid Rain/Deposition:
- Wet Deposition: The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain, snow, fog, or hail.
- Dry Deposition: Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition.
- The acidic particles and gases may deposit to surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can be harmful to human health.
What are the Impacts of Acid Rain?
- Impact on Aquatic Life:
- Acid rain can make water bodies, such as rivers and lakes, inhospitable to certain species of aquatic life such as trout and fish.
- The increased acidity disrupts their reproductive patterns and can lead to fish population decline in affected rivers and lakes.
- Impacts on Marine Water and Species Distribution:
- The increased acidity alters the pH of marine environments, adversely impacting the distribution and survival of various organisms.
- Shell-forming marine species, like mollusks and certain types of plankton, face particular challenges as acidification interferes with their ability to build and maintain protective shells.
- Impacts on Physical Infrastructure:
- Acid rain poses substantial threats to physical structures and monuments, causing deterioration and discoloration.
- Notable examples include the Taj Mahal, whose iconic white marble has been affected, exhibiting a yellowish hue due to sulfuric acid reactions.
- Similarly, buildings, statues, and bridges made of limestone or marble are vulnerable to corrosion and decay.
- Acid rain accelerates the erosion of surfaces, compromising the structural integrity of architectural landmarks.
What are the Acid Rain Mitigation Measures?
- Flue-Gas Desulphurisation:
- Coal power plants have implemented technologies like flue-gas desulphurisation to reduce sulphur dioxide emissions by more than 90%.
- Graded Response Action Plan (GRAP):
- GRAP is a set of emergency measures that kick in to prevent further deterioration of air quality once it reaches a certain threshold in the Delhi-NCR region.
- It was approved by the Supreme Court in the case of M. C. Mehta vs. Union of India 2016 and notified in 2017.
- BS-VI vehicles
- New Commission for Air Quality Management
- Air Quality and Weather Forecasting and Research (SAFAR)
- National Air Quality Index (AQI)
- Air (Prevention and Control of Pollution) Act, 1981
- International Cooperation:
- Governments worldwide collaborate to minimize acid rain, as seen in initiatives such as the Acid Deposition Monitoring Network in East Asia (EANET).
- EANET is an intergovernmental initiative among East Asian countries to monitor and deal with acid deposition, which includes acid rain.
- It collects data on the deposition of acid substances, such as sulfur dioxide and nitrogen oxides, in the atmosphere and their subsequent impact on the environment, particularly ecosystems and water bodies.
- Governments worldwide collaborate to minimize acid rain, as seen in initiatives such as the Acid Deposition Monitoring Network in East Asia (EANET).
What are the Differences Between Acid and Base?
| Characteristic | Acids | Bases |
| Definition | Donate protons (H⁺ ions) | Accept protons (H⁺ ions) or donate pairs of electrons |
| pH on the Scale | Less than 7 (lower pH indicates stronger acid) | Greater than 7 (higher pH indicates stronger base) |
| Ion Formation | Produce hydrogen ions (H⁺) when dissolved in water | Produce hydroxide ions (OH⁻) when dissolved in water |
| Taste | Sour | Bitter |
| Feel (on Skin) | Can be corrosive and may cause a burning sensation | Slippery and soapy feeling |
| Examples | Hydrochloric acid (HCl), sulfuric acid (H₂SO₄) | Sodium hydroxide (NaOH), potassium hydroxide (KOH) |
Way Forward
- There is a need to implement sustainable practices, promoting renewable energy sources, enforcing stringent emissions regulations, fostering international cooperation, and investing in innovative technologies to address environmental challenges and combat climate change.
UPSC Civil Services Examination Previous Year Question (PYQ)
Prelims:
Q1. Why is there a concern about copper smelting plants?
- They may release lethal quantities of carbon monoxide into environment.
- The copper slag can cause the leaching of some heavy metals into environment.
- They may release sulphur dioxide as a pollutant.
Select the correct answer using the code given below.
(a) 1 and 2 only
(b) 2 and 3 only
(c) 1 and 3 only
(d) 1, 2 and 3
Ans: (b)
Exp:
- There are several different processes that can be used for copper production. One of the traditional processes is based on smelting in Reverberatory furnaces (or electric furnaces for more complex ores), producing matte (copper-iron sulphide). The matter from the furnace is charged to converters, where the molten material is oxidized in the presence of air to remove the iron and sulphur impurities (as converter slag) and to form blister copper.
- The principal air pollutants emitted from the process is sulphur dioxide and particulate matter and the main portion of the solid waste is discarded slag. Hence, statement 3 is correct.
- The slag produced can contain significant concentrations of a number of potentially toxic elements including arsenic, lead, cadmium, barium, zinc, etc. The slag can release these potentially toxic elements into the environment under natural weathering conditions and cause pollution of soils, surface waters and groundwater. Hence, statement 2 is correct.
- As slag is considered chemically inert, it is mixed with cement and is used to construct roads and railroad beds. It is also used for sandblasting. Moreover, it is also added to roofing shingles.
- Copper smelting does not release lethal quantities of carbon monoxide into the environment. Hence, statement 1 is not correct.
- Therefore, option (b) is the correct answer.
Q2. With reference to furnace oil, consider the following statements:
- It is a product of oil refineries.
- Some industries use it to generate power.
- Its use causes sulphur emissions into environment.
Which of the statements given above are correct?
(a) 1 and 2 only
(b) 2 and 3 only
(c) 1 and 3 only
(d) 1, 2 and 3
Ans: (d)
Exp:
- Furnace oil or fuel oil is a dark viscous residual product of crude-oil distillation. It is used as a fuel in different types of combustion equipment. The emissions of oxides of sulphur are a direct result of the sulphur content of the fuel oil. Hence, statements 1 and 3 are correct.
- Applications of Furnace oil:
- Marine engines and slow speed engines for power generation,
- Drying tea leaves,
- Gas turbines for power generation,
- Feed stock for fertiliser manufacturing,
- Thermic fluid heaters and hot air generators. Hence, statement 2 is correct.
- Therefore, option (d) is the correct answer.
Mains:
Q1. Describe the key points of the revised Global Air Quality Guidelines (AQGs) recently released by the World Health Organisation (WHO). How are these different from its last update in 2005? What changes in India’s National Clean Air Programme are required to achieve revised standards? (2021)
Q2. Environmental Impact Assessment studies are increasingly undertaken before a project is cleared by the Government. Discuss the environmental impacts of coal-fired thermal plants located at coal pitheads. (2014)