CAR-T Cell Therapy | 12 Feb 2024
Why in News?
Following India’s approval of CAR-T cell therapy, a pioneering treatment for cancer, a patient recently underwent the procedure, achieving freedom from cancer cells, marking a significant advancement in cancer treatment accessibility in the country.
What is CAR-T Cell Therapy?
- About: CAR-T cell therapy, also known as chimeric antigen receptor T-cell therapy, is a type of immunotherapy that uses a patient's own immune system to fight cancer.
- CAR T-cell therapy has been approved for leukaemias (cancers arising from the cells that produce white blood cells) and lymphomas (arising from the lymphatic system).
- CAR-T cell therapies, often referred to as 'living drugs’.
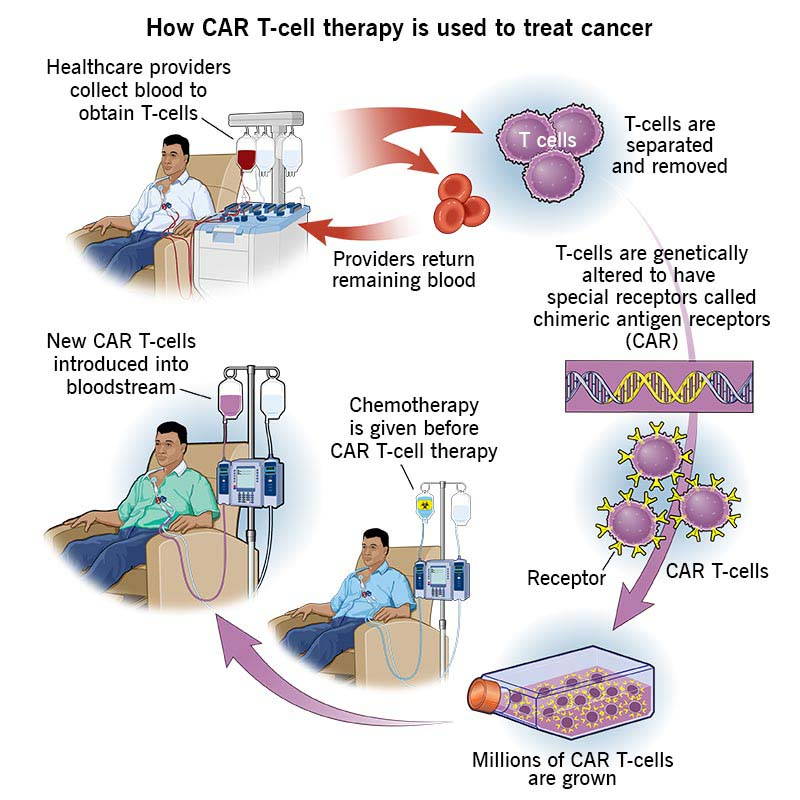
- Procedure: It is a complex and personalised treatment process that involves:
- Collecting T cells: T cells, a type of white blood cell that helps fight infection, are extracted from the patient's blood through a process known as Apheresis.
- Genetic Engineering: In the laboratory, the T cells are genetically modified to express a special protein called a chimeric antigen receptor (CAR) on their surface.
- This CAR is designed to recognize and bind to a specific antigen (marker) found on cancer cells.
- Expansion: The engineered T cells are multiplied in large numbers in the lab.
- Infusion: The expanded CAR-T cells are then infused back into the patient's bloodstream, where they can identify and attack cancer cells that express the targeted antigen.
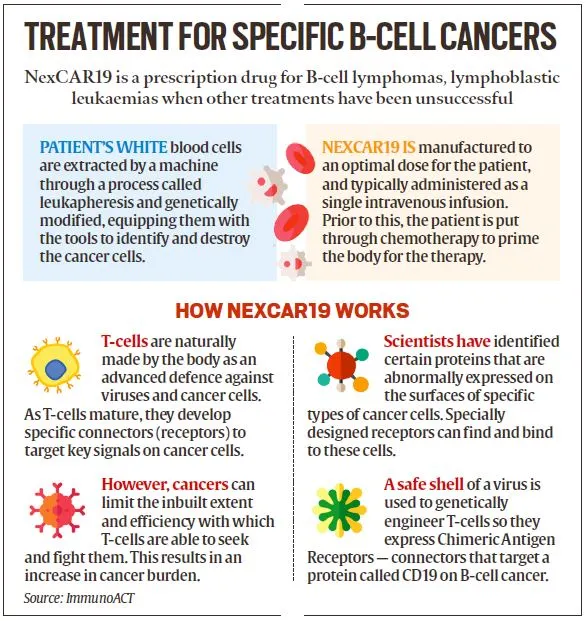
- Development in India: NexCAR19, an indigenously developed therapy for B-cell cancers, has been collaboratively developed by ImmunoACT, Indian Institute of Technology Bombay (IIT-B), and Tata Memorial Hospital.
- The commercial use of this therapy to treat certain blood cancers was approved by the Central Drugs Standard Control Organisation (CDSCO) in October 2023.
- NexCAR19 is the first CAR-T cell therapy to get CDSCO approval.
- Potential Benefits of CAR-T therapy
- High Remission Rates: For some patients with advanced cancers who have not responded to other treatments, CAR-T therapy can lead to high rates of complete remission.
- Personalised Approach: The therapy is tailored to each individual patient's cancer, making it a highly targeted treatment.
- Potential Risks:
- Severe Side Effects: CAR-T therapy can cause serious side effects, including cytokine release syndrome (a widespread activation of the immune system and collateral damage to the body’s normal cells) and neurological symptoms (severe confusion, seizures, and speech impairment).
- High Cost: CAR-T therapy is a very expensive treatment.
What are the Indian Government’s Initiatives Related to Cancer?
- National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke
- National Cancer Grid
- Encouraging Cervical Cancer Vaccination for girls (9-14 years) (Interim Budget 2024-25)
UPSC Civil Services Examination, Previous Year Question (PYQ)
Q. Which one of the following statements best describes the role of B cells and T cells in the human body?(2022)
(a) They protect the environmental allergens. body
(b) They alleviate the body’s pain and inflammation.
(c) They act as immunosuppressants in the body.
(d) They protect the body from diseases caused by pathogens.
Ans: (d)