Science & Technology
India's First CAR-T Cell Therapy Approved
- 16 Oct 2023
- 5 min read
Keywords: CAR-T Cell Therapy, Central Drugs Standard Control Organisation (CDSO), Leukaemia, NexCAR19 (Actalycabtagene autoleucel), T- Cells.
Description: CAR-T Cell Therapy, Developments and their applications and effects in everyday life, Achievements of Indians in science & technology.
Why in News?
Recently, the IIT Bombay-incubated company Immuno Adoptive Cell Therapy has received Central Drugs Standard Control Organisation (CDSO) approval of the first humanized CD19-targeted Chimeric Antigen Receptor T cell (CAR-T cell) Therapy product called NexCAR19 (Actalycabtagene autoleucel) for use in cases of relapsed/refractory B-cell Lymphomas and Leukaemia in India.
- NexCAR19 is a result of a decade-long collaborative effort between IIT Bombay and Tata Memorial Centre (TMC) and has undergone rigorous clinical investigations and translational studies.
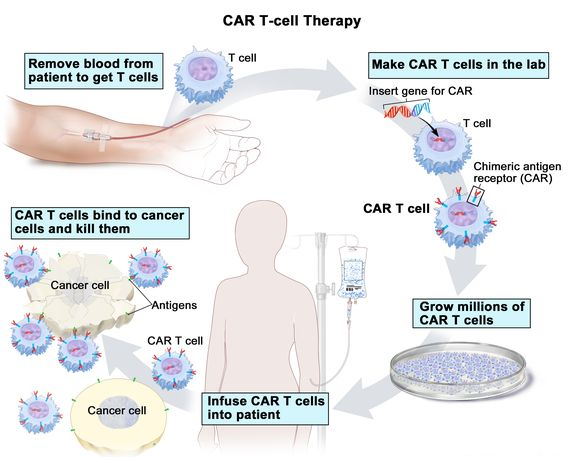
What is CAR T-cell Therapy?
- About:
- CAR T-cell therapies are a major breakthrough in cancer treatment.
- Unlike chemotherapy or immunotherapy which involve taking drugs, CAR T-cell therapies use a patient's own cells. They are modified in the laboratory to activate T-cells and target tumor cells.
- CAR T-cell therapy has been approved for leukaemias (cancers arising from the cells that produce white blood cells) and lymphomas (arising from the lymphatic system).
- CAR T-cell therapies are a major breakthrough in cancer treatment.
- Procedure:
- T cells are taken from a patient’s blood and then the gene for a special receptor that binds to a certain protein on the patient’s cancer cells is added to the T cells in the laboratory.
- The special receptor is called a chimeric antigen receptor (CAR). Large numbers of the CAR T cells are grown in the laboratory and given to the patient by infusion.
- T cells are taken from a patient’s blood and then the gene for a special receptor that binds to a certain protein on the patient’s cancer cells is added to the T cells in the laboratory.
- Significance:
- CAR T-cell therapies are even more specific than targeted agents and directly stimulate the patient's immune system to fight cancer, leading to greater clinical efficacy.
- That's why they're referred to as "living drugs."
- CAR T-cell therapies are even more specific than targeted agents and directly stimulate the patient's immune system to fight cancer, leading to greater clinical efficacy.
- Challenges:
- Preparation: The difficulty of preparing CAR T-cell therapies has been a major hindrance to their widespread use.
- The first successful clinical trial was published a decade ago, and the first indigenously developed therapy in India was performed in 2021.
- Side Effects: In certain kinds of leukaemias and lymphomas, the efficacy is as high as 90%, whereas in other types of cancers it is significantly lower.
- The potential side-effects are also significant, associated with cytokine release syndrome (a widespread activation of the immune system and collateral damage to the body’s normal cells) and neurological symptoms (severe confusion, seizures, and speech impairment).
- Affordability: Introduction of CAR T-cell therapy in India can face challenges of cost and value.
- Critics argue that developing CAR T-cell therapy in India may not be cost-effective as it will still be unaffordable for most people.
- Preparation: The difficulty of preparing CAR T-cell therapies has been a major hindrance to their widespread use.
What are T Cells?
- T cells, also known as T lymphocytes, are a type of white blood cell that play a central role in the immune response.
- T cells are involved in cell-mediated immunity, which means they help the body recognize and respond to foreign substances, such as viruses, bacteria, and abnormal cells, such as cancer cells.
- There are two major types of T cells: the helper T cell and the cytotoxic T cell.
- As the names suggest, helper T cells ‘help’ other cells of the immune system, whilst cytotoxic T cells kill virally infected cells and tumors.
What are the Government Initiatives Related to Cancer Treatment?
UPSC Civil Services Examination, Previous Year Question (PYQ)
Q. Which one of the following statements best describes the role of B cells and T cells in the human body?(2022)
(a) They protect the environmental allergens. body
(b) They alleviate the body’s pain and inflammation.
(c) They act as immunosuppressants in the body.
(d) They protect the body from the diseases caused by pathogens.
Ans: (d)





-min.jpg)