Tropical Ozone Hole
For Prelims: Ozone Layer, Layers of Atmosphere, Ozone Layer Depletion, Greenhouse Gases, Good Ozone, Bad Ozone, Initiatives to tackle Depletion
For Mains: Fundamentals of Atmosphere, Science behind Ozone Layer Depletion, Effects of ozone layer depletion, Related Initiatives
Why in News?
According to a recent study, a new ozone hole has been detected over the tropics, at latitudes of 30 degrees South to 30 degrees North.
What has the Study Revealed?
- The tropical ozone hole is about seven times larger than Antarctica.
- It also appears across all seasons, unlike that of Antarctica, which is visible only in the spring.
- The tropical ozone hole, which makes up 50% of Earth’s surface, could cause a global concern due to the risks associated with it.
- It is likely to cause skin cancer, cataracts and other negative effects on the health and ecosystems in tropical regions.
What do we know about Ozone Layer?
- About:
- It is a special form of oxygen with the chemical formula O3.
- The oxygen we breathe and that is so vital to life on earth is O2.
- Most ozone resides high up in the atmosphere, between 10 and 40 km above Earth's surface. This region is called the stratosphere and it contains about 90% of all the ozone in the atmosphere.
- It is a special form of oxygen with the chemical formula O3.
- Classification:
- Good Ozone:
- Ozone occurs naturally in the Earth's upper atmosphere (Stratosphere) where it forms a protective layer that shields us from the sun's harmful ultraviolet rays.
- This “good” ozone is gradually being destroyed by man-made chemicals referred to as Ozone-Depleting Substances (ODS), including chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), halons, methyl bromide, carbon tetrachloride, and methyl chloroform.
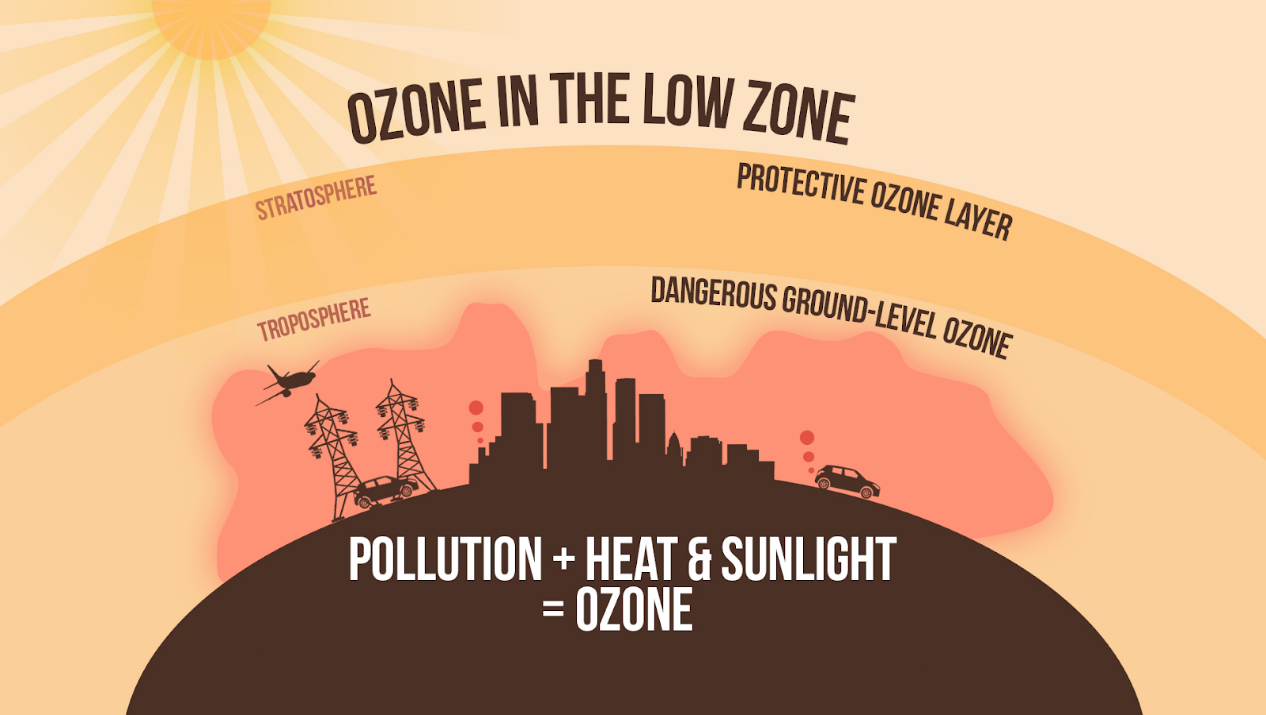
- Bad Ozone:
- In the Earth's lower atmosphere (troposphere) near ground level, ozone is formed when pollutants emitted by cars, power plants, industrial boilers, refineries, chemical plants, and other sources react chemically in the presence of sunlight.
- Surface level ozone is a harmful air pollutant.
- Good Ozone:
Why do we know about Ozone Layer Depletion?
- About:
- Ozone Layer Depletion refers to chemical destruction of the stratospheric ozone layer beyond natural reactions.
- Stratospheric Ozone is constantly being created and destroyed through natural cycles.
- Various Ozone Depleting Substances (ODS), however, accelerate the destruction process, resulting in lower than normal ozone levels.
- ODSs include chlorofluorocarbons (CFCs), bromine-containing halons and methyl bromide, HCFCs, carbon tetrachloride (CCl4), and methyl chloroform.
- These substances were formerly used and sometimes still are used in coolants, foaming agents, fire extinguishers, solvents, pesticides, and aerosol propellants.
- Once released into the air these ozone-depleting substances degrade very slowly.
- In fact, they can remain intact for years as they move through the troposphere until they reach the stratosphere.
- There they are broken down by the intensity of the sun’s UV rays and release chlorine and bromine molecules, which destroy the stratospheric ozone.
- Effects of Depletion:
- On Human Health:
- It increases the amount of UV that reaches the Earth’s surface.
- UV causes non-melanoma skin cancer and plays a major role in malignant melanoma development.
- In addition, UV has been linked to the development of cataracts, a clouding of the eye’s lens.
- It increases the amount of UV that reaches the Earth’s surface.
- On Plants:
- UV radiation affects the physiological and developmental processes of plants. Despite mechanisms to reduce or repair these effects, plant growth can be directly affected by UV radiation.
- Indirect changes caused by UV (such as changes in plant form, how nutrients are distributed within the plant, timing of developmental phases and secondary metabolism) may be equally or sometimes more important than damaging effects of UV.
- On Marine Ecosystem:
- Phytoplankton form the foundation of aquatic food webs. Phytoplankton productivity is limited to the euphotic zone, the upper layer of the water column in which there is sufficient sunlight to support net productivity.
- Exposure to solar UV radiation has been shown to affect both orientation and motility in phytoplankton, resulting in reduced survival rates for these organisms.
- Phytoplankton form the foundation of aquatic food webs. Phytoplankton productivity is limited to the euphotic zone, the upper layer of the water column in which there is sufficient sunlight to support net productivity.
- On Biogeochemical Cycles:
- Increases in UV radiation could affect terrestrial and aquatic biogeochemical cycles, thus altering both sources and sinks of greenhouse and chemically important trace gases (e.g., carbon dioxide, carbon monoxide, carbonyl sulfide, ozone, and possibly other gases).
- On Materials:
- Synthetic polymers, naturally occurring biopolymers, as well as some other materials of commercial interest are adversely affected by UV radiation.
- Increases in UV levels will accelerate their breakdown, limiting the length of time for which they are useful outdoors.
- Synthetic polymers, naturally occurring biopolymers, as well as some other materials of commercial interest are adversely affected by UV radiation.
- On Human Health:
What are the Initiatives for Tackling Ozone Layer Depletion?
- Vienna Convention:
- The 1985 Vienna Convention for the Protection of the Ozone Layer was an international agreement in which United Nations members recognized the fundamental importance of preventing damage to the stratospheric ozone layer.
- India became a Party to the Vienna Convention for the Protection of the Ozone Layer on 18th March 1991.
- Montreal Protocol:
- The 1987 Montreal Protocol on Substances that deplete the Ozone Layer and its succeeding amendments were subsequently negotiated to control the consumption and production of anthropogenic (ODSs) and some hydrofluorocarbons (HFCs).
- India became Party to the Montreal Protocol on substances that deplete the Ozone layer on19th June 1992.
- Kigali Amendment:
- The adoption of the 2016 Kigali Amendment to the Montreal Protocol will phase down the production and consumption of some HFCs and avoid much of the projected global increase and associated climate change.
- EU Regulation:
- EU legislation on ozone-depleting substances is among the strictest and most advanced in the world. Through a series of regulations, the EU has not only implemented the Montreal Protocol but has often phased out dangerous substances faster than required.
- The EU Ozone Regulation sets licensing requirements for all exports and imports of ozone-depleting substances and regulates and monitors not only substances covered by the Montreal Protocol (over 90 chemicals), but also some that are not covered (five additional chemicals called 'new substances').
- India’s regulations for safe use of hydrocarbons as non-ODS alternatives:
- Hydrocarbons including isobutane and cyclopentane are available as non-ODS alternatives for use in aerosols, foam-blowing and refrigeration sectors.
- Safe use of hydrocarbons is regulated by petroleum laws in India.
- The Petroleum Act, 1934 and Petroleum Rules, 1976 relate to handling of a variety of petroleum products.
- The latter also specifies licensing requirements for handling hydrocarbons.
- The Gas Cylinder Rules, 1981, addresses filling, possession, import and transport of cylinders.
UPSC Civil Services Examination, Previous Year Questions (PYQs)
Q. Which one of the following is associated with the issue of control and phasing out of the use of ozone depleting substances? (2015)
(a) Bretton Woods Conference
(b) Montreal Protocol
(c) Kyoto Protocol
(d) Nagoya Protocol
Ans: (b)
Exp:
- The Bretton Woods Conference, officially known as the United Nations Monetary and Financial Conference was a gathering of delegates from 44 nations that met in 1944 in Bretton Woods (USA) to agree upon a series of new rules for the post-World War-II international monetary system.
- The two major accomplishments of the conference were the creation of the International Monetary Fund (IMF) and the International Bank for Reconstruction and Development (IBRD).
- The Montreal Protocol is an international environmental agreement to protect the earth’s ozone layer by eliminating the use of ozone depleting substances. Adopted on 15th September 1987, the protocol is to date the only UN treaty that ever has been ratified by every country on Earth – all 197 UN member states.
- The Kyoto Protocol is an international agreement linked to the UNFCCC, which commits its Parties by setting internationally binding GHGs (Greenhouse Gases) emission reduction targets.
- The Kyoto Protocol was adopted in Kyoto, Japan on 11th December 1997 and entered into force on 16th February 2005.
- The detailed rules for the implementation of the protocol were adopted as CoP7 in Marrakesh, Morocco in 2001 and referred as the Marrakesh Accord.
- India has ratified the second commitment period (2008‑2012) of the Kyoto protocol, that commits countries to contain emissions of greenhouse gases, reaffirming its stand on climate action.
- The Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization provides a transparent legal framework for the effective implementation of one of the three objectives of the Convention on Biological Diversity: the fair and equitable sharing of benefits arising out of the utilization of genetic resources, to promote sustainable use of biological diversity. India signed the protocol in 2011.
- Therefore, option (b) is the correct answer.