-
09 Aug 2024
GS Paper 3
Science & Technology
Day 29: Discuss the potential benefits and challenges associated with CAR-T cell therapy. (150 words)
Approach
- Briefly introduce the CAR-T cell therapy
- Mention potential benefits of the therapy
- Address the issues and challenges associated to CAR-T cell therapy
- Give suggestions to address challenges
- Conclude suitably
Introduction
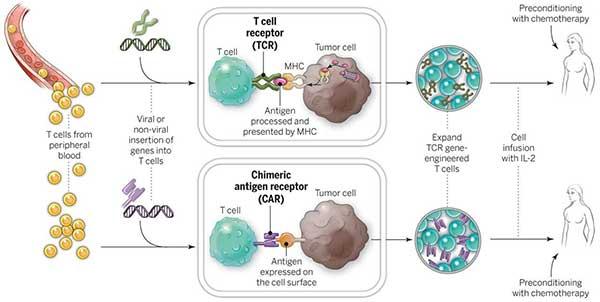
CAR-T cell therapy is an immunotherapy that uses a patient’s modified T-cells to target and kill cancer cells. The process begins with collecting T-cells, then modifying them in a lab to add chimeric antigen receptors (CARs) that help identify cancer cells. After growing the modified T-cells, they are infused back into the patient’s bloodstream to fight the cancer. This therapy has the potential to provide significant benefits, including improved outcomes for patients with certain types of cancer.
Body
Potential Benefits of CAR-T Cell therapy
- Short Treatment Time: CAR T-cell therapy typically involves a single infusion and requires, at most, two weeks of inpatient care.
- Rapid Recovery: Patients recover more quickly compared to stem cell transplants since aggressive chemotherapy is not used.
- Potential to Replace Transplants: In the future, CAR T-cell therapy may replace many types of transplants; currently approved for patients where transplants are not likely to be curative or for those who relapse after a transplant.
- Long-Term Effectiveness: Acts as a “living drug,” with cells persisting in the body for years and potentially recognizing and attacking cancer cells during relapse.
- Durable Remissions: Clinical trials have shown significant remission durations. For example, 42% of adult lymphoma patients were still in remission after 15 months, and two-thirds of children with acute lymphoblastic leukemia were in remission after six months.
Potential Risks and Challenges of CAR-T Therapy:
- Treatment Efficacy and Relapse: CAR-T therapy may not eradicate all cancer cells and might not work for all patients, with some cancers, like large B-cell lymphoma, having higher relapse rates even after initial success.
- Serious Side Effects: The therapy can cause severe side effects, including cytokine release syndrome (immune system activation and damage to normal cells), neurological symptoms (confusion, seizures, speech impairment), and immune effector cell-associated neurotoxicity syndrome (ICANS).
- Preparation Difficulty: The complex preparation process has limited the widespread use of CAR-T therapy, with the first successful clinical trial published a decade ago and the first indigenously developed therapy in India performed in 2021.
- Limited Long-Term Data: There is still limited data on the long-term effects and overall effectiveness of CAR-T therapy.
- Affordability: High costs of CAR-T therapy in India present a significant challenge, raising concerns about its cost-effectiveness and affordability for most people.
Solutions to Address CAR-T Therapy Risks and Challenges:
- Enhance Efficacy and Manage Relapse: Invest in research and combination therapies to improve effectiveness and reduce relapse rates.
- Mitigate Serious Side Effects: Implement rigorous monitoring and develop targeted treatments for managing severe side effects like ICANS and cytokine release syndrome.
- Simplify Preparation and Increase Data: Optimize preparation processes, standardize procedures, and support long-term studies for better understanding and efficiency.
- Improve Affordability: Develop cost-reduction strategies and work with insurers to expand coverage, making CAR-T therapy more accessible.
Conclusion
CAR-T cell therapy offers significant benefits, such as targeting cancer cells with high precision, potentially leading to durable remissions and acting as a “living drug” that can persist long-term. The introduction of NexCAR19, India’s first indigenously developed and most affordable CAR-T therapy, addresses some of these challenges by making advanced treatment more accessible and cost-effective. NexCAR19 represents a major advancement in global cell and gene therapy, positioning India as a leader in innovative cancer treatments.