Antimicrobial Resistance: The Urgent Call for Action | 08 Oct 2024
This editorial is based on “Virtuous viruses to fight antimicrobial resistance” which was published in Hindustan Times on 07/10/2024. The article brings into picture the rising importance of bacteriophages as a solution to antimicrobial resistance, highlighting their ability to target drug-resistant bacteria and offering a potential alternative to failing antibiotics. It emphasizes the urgency of phage therapy, particularly for countries like India, facing critical drug resistance challenges.
For Prelims: Bacteriophages, Antimicrobial Resistance, Antibiotics, Central Drug Regulator, E. coli and Klebsiella pneumoniae, 2019 Colistin ban, AMR Surveillance Network, One Health approach, Schedule H1 of the Drugs and Cosmetics Rules, Ayushman Arogya Mandir, Global Antimicrobial Resistance Surveillance System.
For Mains: Factors Driving the Growth of AMR in India, Indian Government Initiatives to Tackle AMR.
Bacteriophages, or "phages," are emerging as a promising solution to combat the growing threat of antimicrobial resistance. These viruses, which naturally prey on bacteria, have the potential not only to fight drug-resistant bacteria but also to lower resistance in them. Phages work by invading bacteria, seizing their genetic material, and destroying them from within. Their ubiquity in nature, from wastewater to the human gut, makes them an attractive option for therapeutic development.
The urgency of exploring phage therapy is underscored by the looming crisis of antimicrobial resistance, which is predicted to cause 40 million deaths by 2050. Recent scientific breakthroughs have demonstrated the ability of phages to reverse antibiotic resistance in bacteria like Pseudomonas aeruginosa, a common cause of life-threatening hospital-acquired infections. As conventional antibiotics lose their efficacy, countries worldwide are intensifying their search for therapeutic phages. For nations like India, facing serious drug resistance problems, phage therapy could offer a crucial alternative in the fight against superbugs.
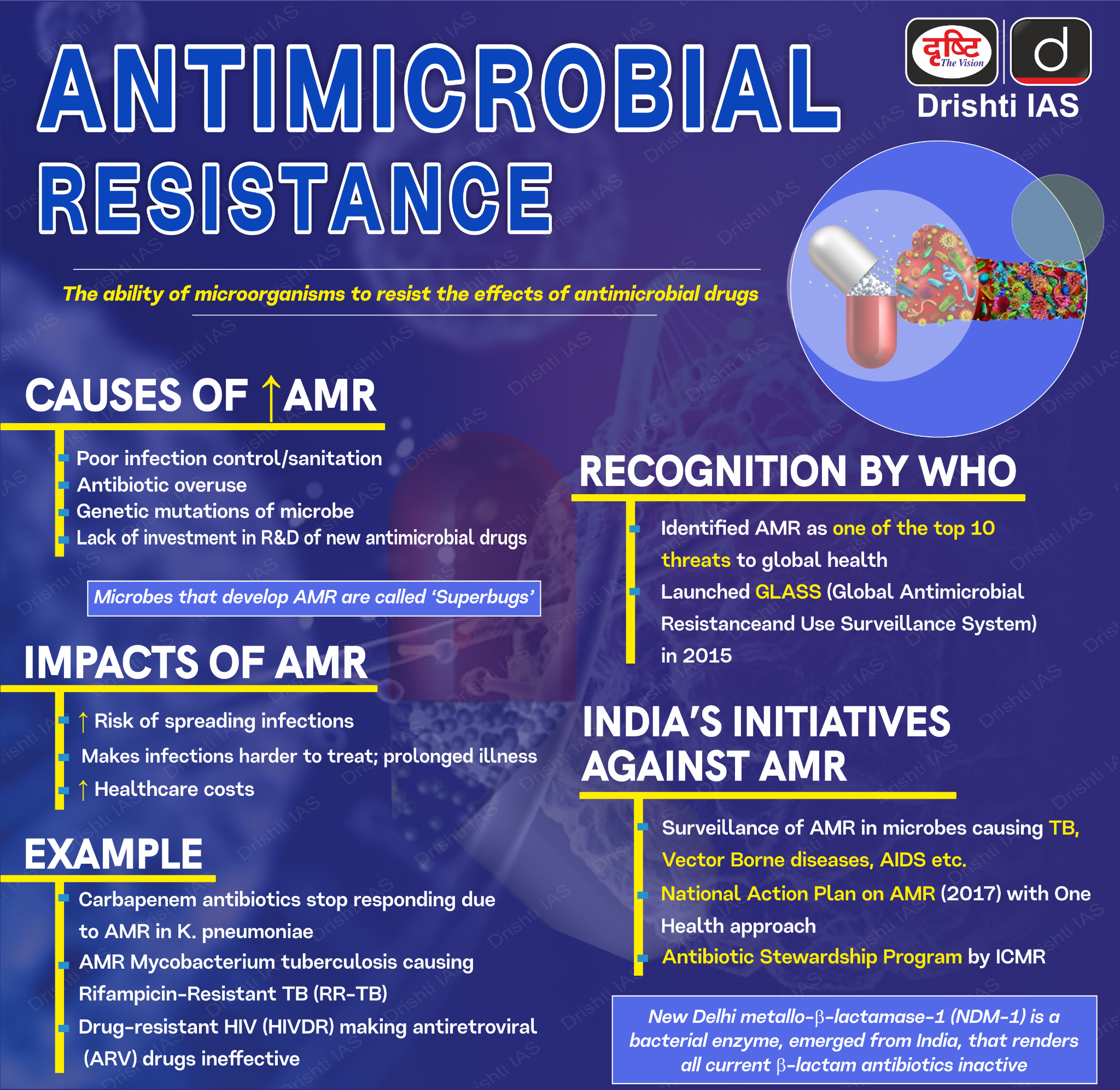
What is Antimicrobial Resistance?
- Antimicrobial Resistance (AMR) refers to the ability of microorganisms such as bacteria, viruses, fungi, and parasites to resist the effects of medications that were once effective against them, including antibiotics, antivirals, antifungals, and antiparasitics.
- As a result, standard treatments become ineffective, infections persist, and they may spread to others, increasing the risk of severe illness, disability, and death.
- According to WHO, AMR is a top global public health threat, directly responsible for 1.27 million deaths in 2019 and contributing to 4.95 million deaths.
- The World Bank estimates AMR could add USD 1 trillion in healthcare costs and cause GDP losses between USD 1 trillion and USD 3.4 trillion annually by 2030.
What are the Factors Driving the Growth of AMR in India?
- Overuse and Misuse of Antibiotics: Antibiotics in India are widely available over-the-counter, and there is a tendency among both healthcare providers and the general public to over-prescribe or misuse them.
- According to 2022 Lancet Study, more than 47% of antibiotic formulations used in India's private sector in 2019 were not approved by the Central Drug Regulator
- This unchecked availability has led to widespread, often unnecessary use.
- A recent government survey revealed that over 38% of inpatients in Indian hospitals are prescribed multiple antibiotics, with more than 55% of these prescriptions belonging to the WHO's "Watch" group, which is reserved for severe infections.
- In addition, a WHO Global Systematic Review showed that although only 6% of 76,176 COVID-19 cases reviewed had bacterial or fungal co-infections, 62% received antibiotics
- This overuse promotes resistance, allowing bacteria to evolve and withstand standard treatments.
- According to 2022 Lancet Study, more than 47% of antibiotic formulations used in India's private sector in 2019 were not approved by the Central Drug Regulator
- Inadequate Infection Control and Hygiene Practices in Healthcare Settings: Poor infection control practices in healthcare facilities contribute significantly to AMR in India.
- High rates of hospital-acquired infections (9.06 infections per 1,000 intensive care unit (ICU) patient days), often involving drug-resistant bacteria, are prevalent.
- Hospitals, particularly in public sectors with resource constraints, sometimes lack the infrastructure to enforce stringent infection control measures.
- For instance, studies have shown that multi-drug resistant infections in Indian hospitals are increasing, with reports of resistant strains of E. coli and Klebsiella pneumoniae in intensive care units (ICUs).
- Use of Antibiotics in Agriculture and Animal Husbandry: In India, antibiotics are commonly used in agriculture to promote growth and prevent disease in animals, contributing to AMR when residues enter the human food chain.
- The agricultural sector’s unregulated antibiotic use is a major concern, with residues found in poultry and dairy products.
- In a recent study by the Center for Science and Environment, residues of antibiotics were found in the liver, muscle and kidney tissues of chicken samples.
- The Indian government has made strides to regulate this through guidelines for antibiotic use in food-producing animals, like center has recommended to ban chloramphenicol and nitrofurans in food-producing animals following the 2019 Colistin ban, yet enforcement remains a challenge, and practices continue in many regions .
- Environmental Contamination by Pharmaceutical Waste: India is one of the world’s largest producers of generic drugs, including antibiotics.
- However, lax regulations around pharmaceutical waste disposal have led to significant environmental contamination.
- It can be easily found in areas around pharmaceutical manufacturing hubs, such as antibiotics from the fluoroquinolone group found in the Musi River, in Hyderabad, India, promoting the growth of resistant bacteria in the environment.
- Challenges in Pharmaceutical Quality Control: The pharmaceutical sector’s rapid expansion has often outpaced regulatory oversight, resulting in substandard antibiotic production.
- Poor-quality antibiotics either due to inadequate active ingredients or contamination contribute to resistance as they fail to effectively kill bacteria.
- Recognizing this issue, the Indian government set a 6 month and 12-month deadline in 2023 for pharmaceutical companies to adhere to WHO Good Manufacturing Practices.
- However, given the scale of the industry, compliance remains uneven, highlighting the need for continued regulatory vigilance
- Lack of Public Awareness: Public understanding of AMR remains limited, especially in rural areas. Many patients prematurely stop antibiotic courses or use them improperly, unaware of the long-term consequences.
- According to a community-based survey, 24% of participants were unaware of the consequences of increasing antimicrobial resistance (AMR) levels. (Observer Research Foundation)
- In response, states like Kerala have begun forming local committees to educate communities on the importance of responsible antibiotic use.
- Despite these efforts, the broader population’s lack of awareness continues to fuel AMR.
What are the Indian Government Initiatives to Tackle AMR?
- AMR Surveillance Network: Strengthened with labs in State Medical Colleges, covering 36 sites across 26 States/UTs (As of August 2022).
- ICMR's AMR Surveillance and Research Network monitors drug-resistant infections in 30 tertiary care hospitals (both private and government).
- National Action Plan on AMR: Launched in 2017 with a One Health approach, involving multiple ministries.
- Delhi Declaration on AMR signed by ministers, pledging support for containment efforts.
- Research & International Collaboration: ICMR partnered with Norway and Germany for AMR research and new drug development.
- Awareness and Regulation:
- 40 fixed-dose combinations (FDCs) banned by the Drugs Controller General of India.
- Collaboration with agriculture and animal husbandry departments to ban Colistin in poultry feed.
- Awareness campaigns through schools, colleges, and public platforms focus on proper antibiotic use and hand hygiene.
What Measures can India Adopt to Contain the Rise of AMR?
- Strengthen Antibiotic Stewardship Programs in Healthcare Settings: Implement mandatory antibiotic stewardship programs in all hospitals, following the National Guidelines for Infection Prevention and Control in Healthcare Facilities 2020.
- These programs should include regular audits of antibiotic prescriptions, feedback to prescribers, and continuous education for healthcare professionals.
- Utilise digital health technologies, such as e-Sanjeevani telemedicine platform, to provide real-time guidance on appropriate antibiotic use to healthcare providers across the country.
- Encourage the use of rapid diagnostic tests to reduce empirical antibiotic prescriptions. For instance, the Indian startup Module Innovations has developed a rapid test for urinary tract infections that can guide antibiotic selection, potentially reducing unnecessary broad-spectrum antibiotic use.
- Enforce Regulations on Over-the-counter Antibiotic Sales: Strengthen the implementation of Schedule H1 of the Drugs and Cosmetics Rules, which restricts the sale of certain antibiotics without prescription.
- Introduce a digital tracking system for antibiotic sales, similar to the e-pharmacy model proposed in the Draft Drugs and Cosmetics Amendment Rules, 2023.
- This system could help monitor antibiotic dispensing patterns and flag unusual sales.
- Conduct regular inspections of pharmacies and impose stricter penalties for non-compliance.
- Launch public awareness campaigns about the risks of self-medication with antibiotics, leveraging popular media and community health workers.
- Regulate Antibiotic use in Agriculture and Animal Husbandry: Fully implement the National Action Plan on AMR (2022-2026) measures related to phasing out antibiotics as growth promoters in animals.
- Establish a robust surveillance system for antibiotic use in agriculture, similar to the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) program.
- Promote alternatives to antibiotics, such as probiotics and improved animal husbandry practices.
- Improve Wastewater Treatment in Pharmaceutical Manufacturing: Enforce stricter environmental regulations on pharmaceutical manufacturing, including mandatory advanced wastewater treatment technologies.
- Introduce a "green pharmacy" certification for antibiotic manufacturers who meet stringent environmental standards, similar to the EU's Good Manufacturing Practice (GMP) certification.
- Collaborate with industry leaders like Dr. Reddy's Laboratories, which has implemented zero liquid discharge systems in 88% of its facilities in India, to develop best practices for the sector.
- Invest in research on innovative wastewater treatment technologies, such as the advanced oxidation processes for pharmaceutical effluent treatment.
- Enhance Infection Prevention and Control Measures: Introduce regular infection control audits and link them to hospital accreditation processes.
- Invest in infrastructure improvements to reduce overcrowding and improve sanitation in healthcare facilities, utilizing initiatives like Ayushman Arogya Mandir.
- Implement nationwide hand hygiene campaigns in healthcare settings and promote the use of hand sanitizers and ensure their availability at all points of care, leveraging India's capacity for low-cost production of these products.
- Expand and Strengthen AMR Surveillance: Rapidly scale up the ICMR's AMR surveillance network to cover more sites. Integrate AMR surveillance with existing disease surveillance programs, such as the Integrated Disease Surveillance Programme (IDSP).
- Implement the One Health approach to AMR surveillance, including environmental and animal health sectors, following the model of the recently established One Health Support Unit under the Department of Animal Husbandry and Dairying.
- Utilise advanced genomic surveillance techniques, such as whole-genome sequencing, to track the emergence and spread of resistant pathogens.
- Collaborate with international initiatives like the Global Antimicrobial Resistance Surveillance System (GLASS) to standardise data collection and reporting methodologies.
Conclusion:
Tackling antimicrobial resistance in India requires a multifaceted approach, including the adoption of bacteriophage therapy as a promising alternative to conventional antibiotics. Strengthening regulatory frameworks, enhancing public awareness, and implementing effective infection control measures are crucial to curbing the rise of AMR. By fostering collaboration across sectors and prioritising research, India can address this growing public health threat.
|
Drishti Mains Question: Antimicrobial Resistance poses a significant threat to public health in India, with implications for healthcare systems, food security, and economic development. Examine the government's efforts to tackle AMR and discuss what more can be done to strengthen India's response to this growing challenge? |
UPSC Civil Services Examination, Previous Year Questions (PYQ)
Prelims
Q. Which of the following are the reasons for the occurrence of multi-drug resistance in microbial pathogens in India? (2019)
- Genetic predisposition of some people
- Taking incorrect doses of antibiotics to cure diseases
- Using antibiotics in livestock farming
- Multiple chronic diseases in some people
Select the correct answer using the code given below.
(a) 1 and 2
(b) 2 and 3 only
(c) 1, 3 and 4
(d) 2, 3 and 4
Ans: (b)
Mains
Q. Can overuse and free availability of antibiotics without Doctor’s prescription be contributors to the emergence of drug-resistant diseases in India? What are the available mechanisms for monitoring and control? Critically discuss the various issues involved. (2014)