Important Facts For Prelims
World's Oceans Approaching Critical Acidification Level
- 27 Sep 2024
- 5 min read
Why in News?
Recently, a recent report released by the Potsdam Institute for Climate Impact Research (PIK), Germany highlighted an alarming trend regarding ocean acidification.
- It indicated that the world’s oceans are nearing a critical threshold that could severely impact marine life and climate stability.
What are the Key Findings of the Report?
- Planetary Boundaries: Six of nine critical Earth systems, including climate change, biodiversity loss, and pollution, have been breached.
- Ocean Acidification: Rising CO₂ emissions are set to exceed sustainable levels of ocean acidification.
- Tipping Points and Potential Recovery: Crossing ecological tipping points threatens irreversible ecosystem damage and impacts billions. Although the ozone layer is recovering, urgent action is required on other environmental boundaries to prevent further degradation.
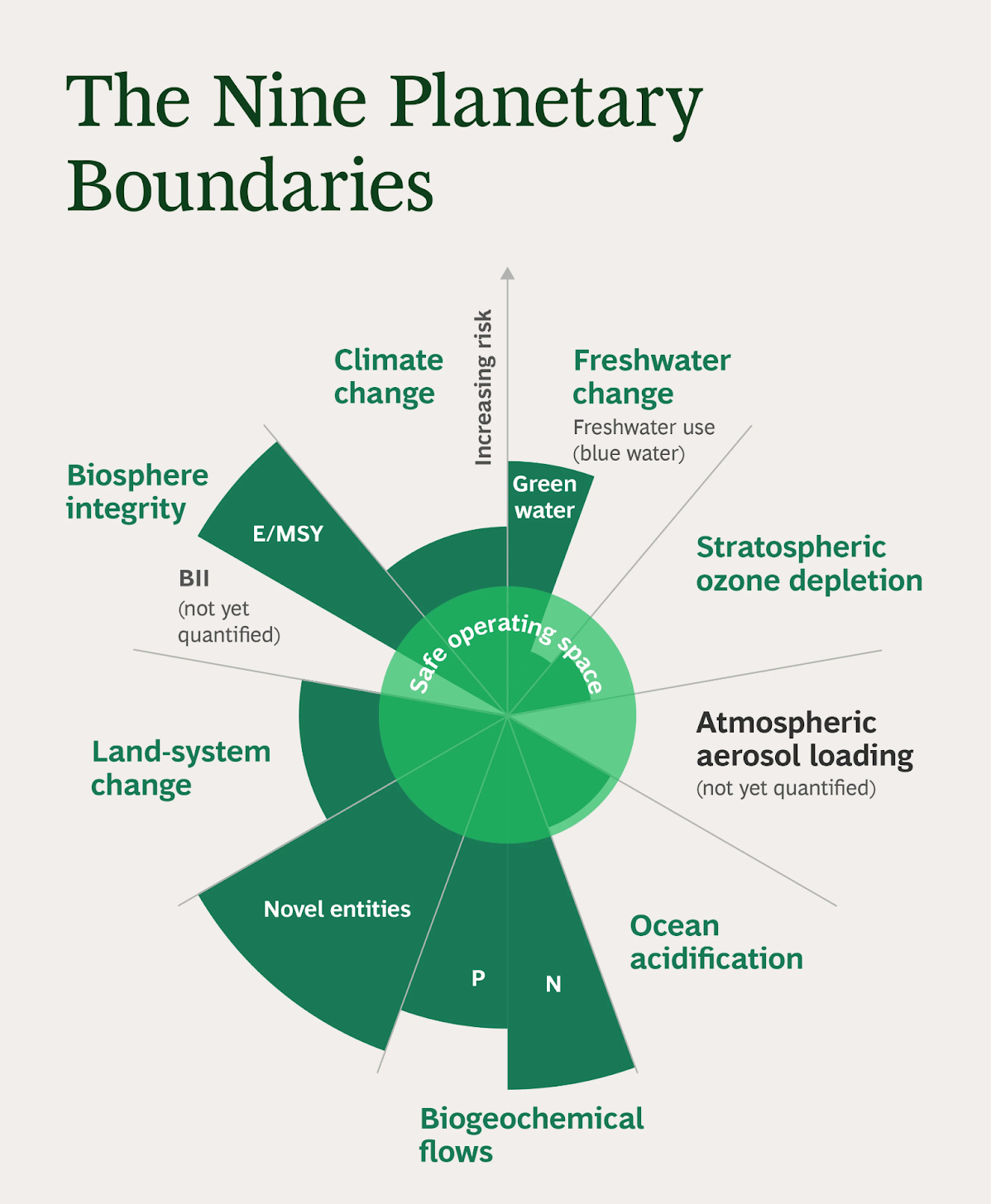
Planetary Boundaries
- About:
- The planetary boundaries framework, introduced by Johan Rockström and 28 scientists in 2009, outlines earth's environmental limits to ensure stability and biodiversity for humanity's safe operation.
- Nine Planetary Boundaries:
- Climate change.
- Change in biosphere integrity (biodiversity loss and species extinction)
- Stratospheric ozone depletion.
- Ocean acidification.
- Biogeochemical flows (phosphorus and nitrogen cycles).
- Land-system change (for example deforestation).
- Freshwater use (alterations across the entire water cycle over land).
- Atmospheric aerosol loading (microscopic particles in the atmosphere that affect climate and living organisms).
- Introduction of novel entities(consisting of microplastics, endocrine disruptors, and organic pollutants).
- Breaching Planetary Boundaries:
- Breaching planetary boundaries doesn't signal an immediate disaster but increases the risk of irreversible environmental shifts, potentially making Earth uninhabitable for our current way of life.
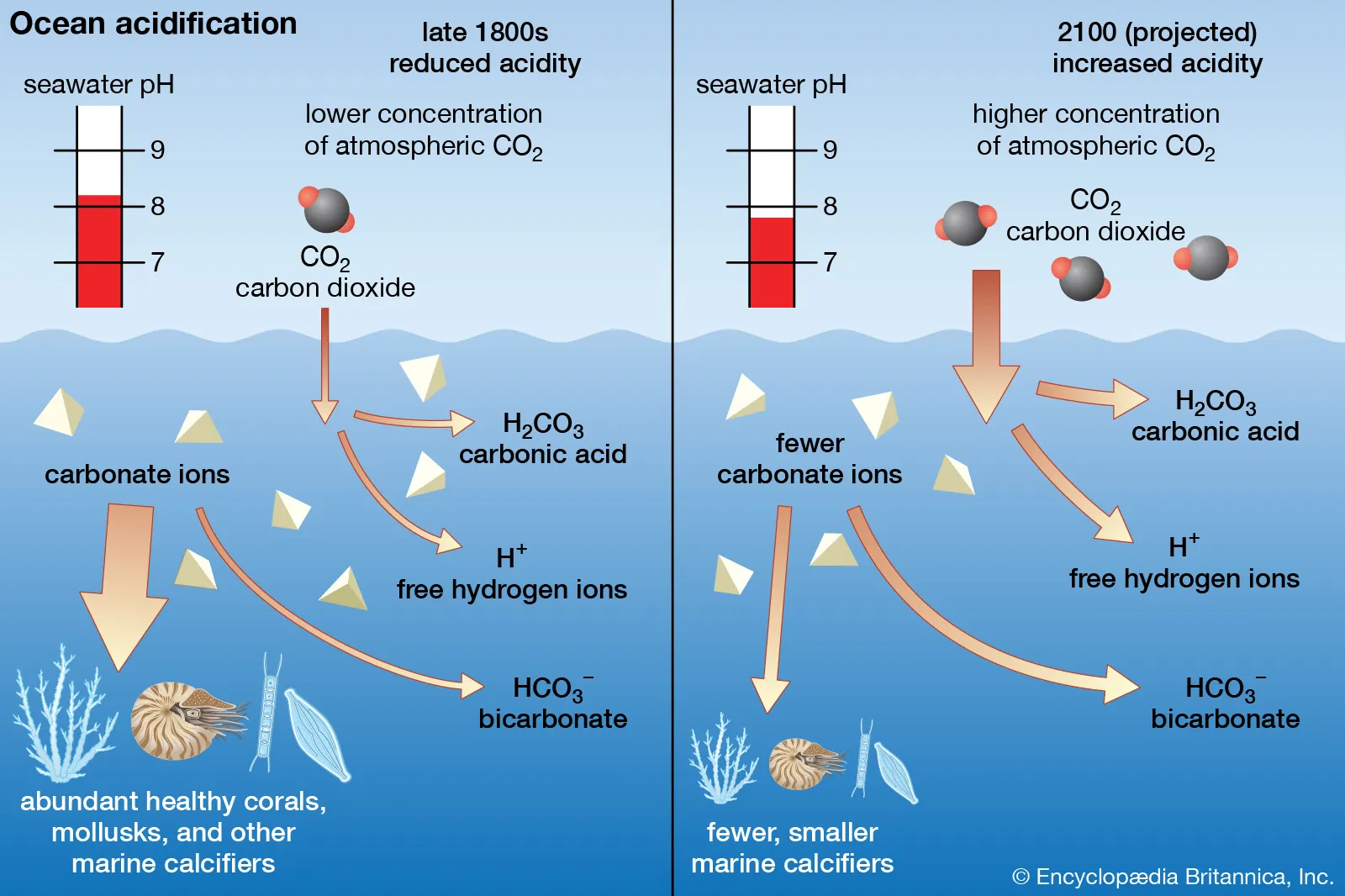
What is Ocean Acidification?
- About:
- It refers to the process by which the pH levels of the ocean decrease due to the absorption of excess atmospheric CO₂.
- As CO₂ levels rise, more of it dissolves in seawater, forming carbonic acid, which lowers the pH.
- Ocean Acidification Process:
- When CO2 is absorbed by seawater, it triggers chemical reactions that raise hydrogen ions (H+) concentrations.
- CO2 dissolves and forms carbonic acid (H2CO3), which breaks down into hydrogen ions (H+) and bicarbonate ions (HCO3-).
- The rise in H+ increases the acidity of seawater, reducing the availability of carbonate ions.
- Climate Change Accelerating Ocean Acidification:
- Oceans naturally absorb carbon dioxide, but increased atmospheric carbon dioxide levels have caused oceans to absorb too much CO2, leading to a nearly 30% increase in acidity since the 1800s, about 10 times faster than in the last 50 million years.
- If emissions continue, surface ocean pH could drop from 8.1 to 7.7 in the next 100 years.
- Coastal areas are especially vulnerable due to acid sulphate runoff, and climate change-related sea level rise worsens these effects.
- Impact of Ocean Acidification:
- This change in acidity can have detrimental effects on marine organisms, particularly those with calcium carbonate shells or skeletons, such as corals and shellfish.
UPSC Civil Services Examination, Previous Year Question (PYQ)
Prelims
Q. The acidification of oceans is increasing. Why is this phenomenon a cause of concern? (2012)
- The growth and survival of calcareous phytoplankton will be adversely affected.
- The growth and survival of coral reefs will be adversely affected.
- The survival of some animals that have phytoplanktonic larvae will be adversely affected.
- The cloud seeding and formation of clouds will be adversely affected.
Which of the statements given above is/are correct?
(a) 1, 2 and 3 only
(b) 2 only
(c) 1 and 3 only
(d) 1, 2, 3 and 4
Ans: (a)