Science & Technology
Side-Effects of Covid-19 Vaccine
- 11 May 2024
- 8 min read
For Prelims: Vaccines and types, Virus Strain and Mutation, Covishield and Covaccine, World Health Organisation(WHO).
For Mains: Reasons for Land subsidence, and Measures and Recommendations.
Why in News?
Recently, there has been a lot of controversy over the side-effects of Oxford-AstraZeneca’s vaccine. It is sold in India under the brand name of “Covishield” by Serum Institute of India (SII)
- It is being linked to a rare adverse side effect called Thrombosis with Thrombocytopenia Syndrome (TTS).
What is Thrombocytopenia Syndrome?
- About:
- TTS is also referred to as vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) or vaccine-induced immune thrombotic thrombocytopenia (VITT).
- This rare syndrome has been observed in individuals who have received Covid-19 vaccines utilising adenoviral vectors.
- It is generally believed to be caused by an immune reaction triggered by the adenovirus vector used in these vaccines.
- Adenoviruses are non-enveloped, double-stranded DNA viruses which are considered excellent vectors for delivering target antigens to mammalian hosts because of their capability to induce both innate and adaptive immune responses.
- Symptoms:
- TTS is linked to a variety of symptoms such as difficulty breathing, chest or limb pain, small red spots or bruising beyond the injection site, headaches, numbness in body parts, and more.
- Thrombosis refers to the formation of blood clots, while thrombocytopenia is characterised by a low platelet count.
- TTS is linked to a variety of symptoms such as difficulty breathing, chest or limb pain, small red spots or bruising beyond the injection site, headaches, numbness in body parts, and more.
- Risk- Benefit Analysis:
- Risk:
- TTS most commonly occurs in healthy young women around thirty years old at a low frequency of about one to two cases per 100,000.
- At a general population level, it is estimated to occur at only about two to three cases per million vaccinated people.
- The annual risk of TTS is still much lower than the annual risk of dying in a road accident.
- TTS most commonly occurs in healthy young women around thirty years old at a low frequency of about one to two cases per 100,000.
- Benefit:
- Covishield has shown over 80% protection against severe COVID-19 and over 90% protection against death in various studies, even during the Delta wave.
- For a 50% chance of getting Covid-19 and a 0.1% risk of death, the vaccine provides a significant mortality benefit, outweighing the risks by far.
- It has not only reduced disease severity and minimised immediate suffering and stress on healthcare systems but also to lower the risk of long-term disabilities and premature heart attacks and strokes.
- This risk was noted early in the pandemic, before vaccines were available, and vaccination has been shown to reduce this risk.
- Other Rare Side Effects of Covid-19 Vaccines:
- A study of 99 million people found that cases of Guillain Barre Syndrome, myocarditis, pericarditis, and cerebral venous sinus thrombosis (CVST) were at least 1.5 times higher than expected after receiving mRNA and ChAdOX1 (or Covishield) vaccine for Covid-19.
- The study confirmed that these illnesses were classified as ‘rare’ side effects following the vaccination for Covid-19.
- CVST refers to cerebral venous sinus thrombosis, which is the presence of blood clots in the brain.
- Guillain-Barre syndrome is an immune system disorder that attacks the nerves, causing muscular damage and requiring prolonged treatment.
- Myocarditis and pericarditis are conditions involving inflammation of the heart tissue.
- Risk:
What were the Regulations and Concerns Related to Covid-19 Vaccination in India?
- Regulations Related to Covid-19 Vaccines in India:
- India has used nearly 1.75 billion doses to vaccinate approximately 80% of its vaccinated population.
- Covid-19 vaccines were administered without the completion of phase-3 trials, and the manufacturers did not have complete information on possible short-term or long-term side effects or fatalities.
- E.g. The Phase 3 protocol for Covaxin (by Bharat Biotech) was approved before the completion of Phase 2, and the final vaccine candidate was chosen without considering the Phase 2 trial data.
- The Corbevax vaccine (by Biological E) received emergency use authorisation from the Drug Controller General of India (DCGI) for vaccinating 12-14-year-old children.
- Concern Related to Covid-19 Vaccines:
- In March 2021, Several European countries temporarily paused the use of AstraZeneca's vaccine due to reported cases of blood clotting.
- The World Health Organization stated that TTS was being reported in some cases after vaccinations with Covishield and Vaxzevria, but emphasised that the risk appears to be very low based on available data.
- European nations, UK, USA, and Australia halted the use of Covieshield due to TTS reports, despite the benefits outweighing the risks.
- They had enough mRNA (like Pfizer-BioNTech and Moderna Covid-19) vaccines available, which were more immunogenic and not linked to TTS, although cases of non-fatal myocarditis had been seen.
- In 2023, the WHO included vaccine-induced immune thrombotic thrombocytopenia (VITT) in its classification of thrombosis with thrombocytopenia syndrome (TTS).
- India’s Stand:
- Before the Covid-19 vaccines were rolled out in India, the Indian government issued a fact sheet in January 2021 cautioning the use of Covishield for individuals with low platelet counts.
- In May 2021, the Indian government reported 26 potential cases of blood clots related to the Covishield vaccine, with a rate of 0.61 cases per million doses.
- The government maintained that the risk is minimal and that Covishield has a positive benefit-risk profile. No such events were reported for the indigenous vaccine, Covaxin (by Bharat Biotech).
- The Ministry of Health and Family Welfare, GoI also noted that the risk of blood clotting is lower in individuals of South and Southeast Asian descent compared to those of European descent.
FLiRT- A New Variant of Covid-19
- It is a new variant within the Omicron JN.1 lineage.
- It has been detected in the US and is rapidly spreading.
- This variant shows significant alterations in spike (S) protein structure and increased resistance to existing vaccines.
- Its symptoms are similar to Omicron, including sore throat, cough, congestion, tiredness, headache, muscle or body aches, runny nose, fever or chills, loss of smell and taste, and breathlessness in extreme cases.
- This variant is highly transmissible and can spread via respiratory droplets or touching infected surfaces.
UPSC Civil Services Examination Previous Year Questions (PYQs)
Prelims:
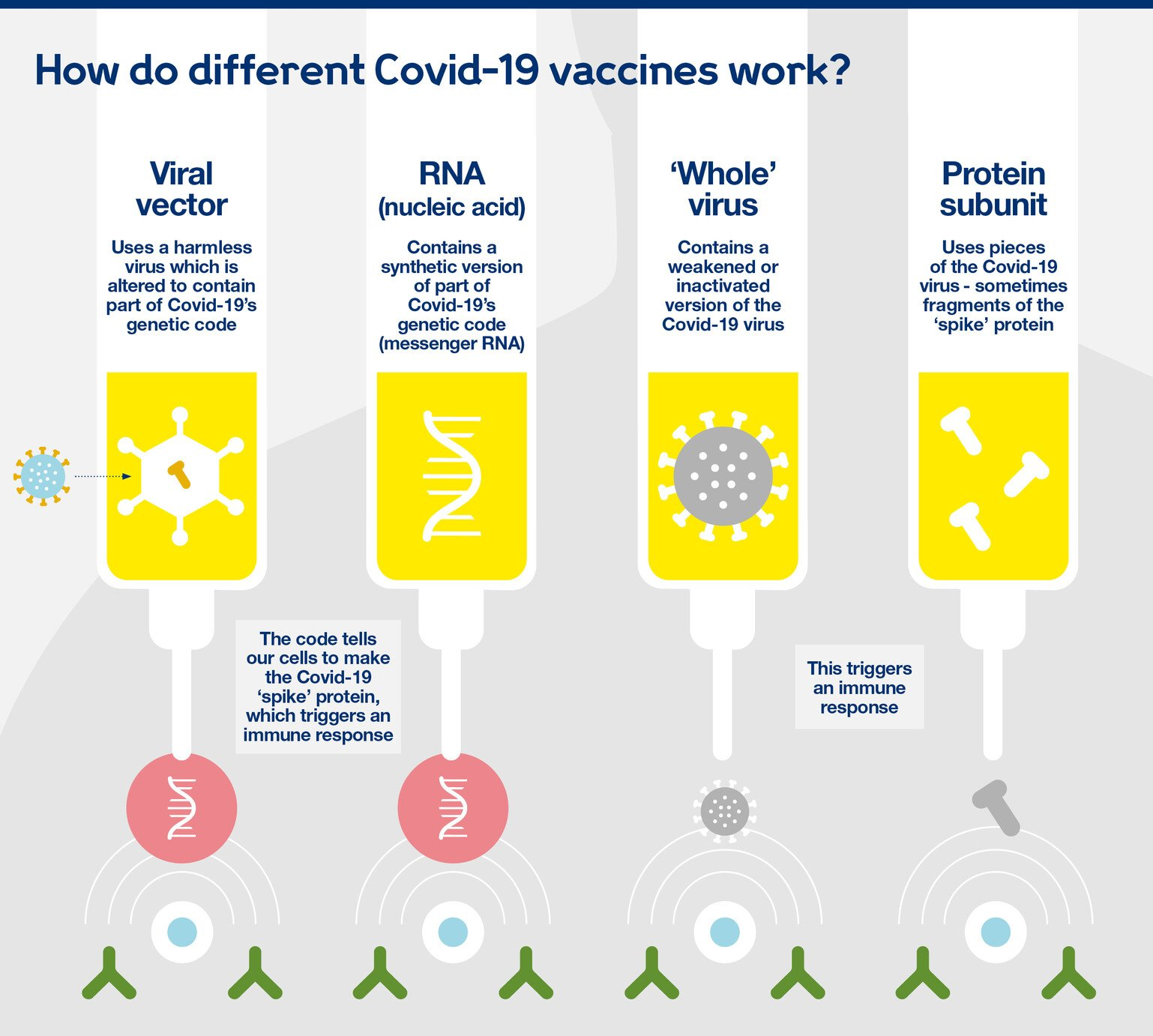
Q. In the context of vaccines manufactured to prevent COVID-19 pandemic, consider the following statements: (2022)
- The Serum Institute of India produced COVID-19 vaccine named Covishield using mRNA platform.
- Sputnik V vaccine is manufactured using a vector-based platform.
- COVAXIN is an inactivated pathogen-based vaccine.
Which of the statements given above are correct?
(a) 1 and 2 only
(b) 2 and 3 only
(c) 1 and 3 only
(d) 1, 2 and 3
Ans: (b)