Science & Technology
Sickle Cell Disease
- 28 Mar 2024
- 10 min read
For Prelims: Biotechnology, Sickle Cell disease, Genetic disorder, Tribal communities
For Mains: Challenges and Governmental Initiatives faced by the tribal communities relating to the treatment and accessibility of sickle cell disease (SCD)
Why in News?
Amidst the unavailability of essential drugs to treat Sickle Cell Disease (SCD) at district healthcare institutions, there is growing concern about the challenges faced by people from marginalised Indigenous Tribal communities in managing the treatment of SCD.
What is Sickle-Cell Disorder?
- About:
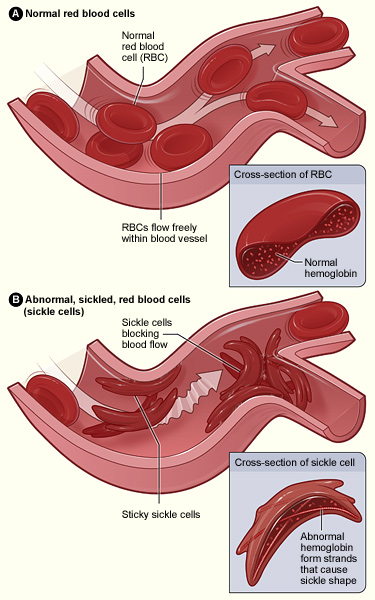
- Sickle Cell Disease (SCD) is an inherited haemoglobin disorder characterised by a genetic mutation that causes red blood cells (RBCs) to assume a sickle or crescent shape rather than their normal round shape.
- This abnormality in RBCs results in increased rigidity, impairing their ability to circulate effectively throughout the body. Consequently, individuals with SCD often experience complications such as anaemia, organ damage, recurrent and severe pain episodes, and a shortened lifespan.
- As per the Ministry of Health and Family Welfare, marginalised tribal populations are most vulnerable to SCD.
- Symptoms: Symptoms of sickle cell disease can vary, but some common symptoms are-
- Chronic anaemia which leads to fatigue, weakness, and paleness.
- Painful episodes (also known as sickle cell crisis) cause sudden and intense pain in the bones, chest, back, arms, and legs.
- Delayed growth and puberty.
- Treatment Processes:
- Blood Transfusions: These can help relieve anaemia and reduce the risk of pain crises.
- Hydroxyurea: This medication can help reduce the frequency of painful episodes and prevent some of the disease's long-term complications.
- Gene Therapy: It can also be treated by bone marrow or stem cell transplantation by methods like Clustered regularly interspaced short palindromic repeats (CRISPR).
What is India's Current Status of Sickle Cell Disease (SCD)?
- India ranks third globally in terms of the number of SCD births, following Nigeria and the Democratic Republic of the Congo.
- Regional studies indicate that an estimated 15,000 to 25,000 babies with SCD are born in India annually.
- Most of these births occur in tribal communities, highlighting the geographical and socioeconomic disparities in healthcare access and awareness.
What are the Challenges Related to the Treatment and Accessibility of SCD?
- Limited Awareness: There is a lack of understanding about SCD among the public and healthcare providers, leading to delayed diagnosis and inadequate treatment.
- Inadequate Healthcare Infrastructure: Many rural and tribal areas lack specialised healthcare facilities and trained medical personnel for managing SCD.
- High Treatment Costs: The long-term management of SCD can be financially burdensome for many families due to the cost of medications, regular check-ups, and potential hospitalizations.
- For Example, Treatments like CRISPR cost $ 2-3 million, and it's difficult to find bone marrow donors.
- Limited Access to Medications: Inconsistent availability of essential medications for SCD treatment, such as hydroxyurea and pain relievers, is a concern in certain regions.
- Inadequate Screening Programs: The absence of systematic newborn screening and early detection initiatives results in missed opportunities for early intervention and genetic counselling.
- Geographical and Socioeconomic Barriers: Rural, remote, and tribal communities face challenges in accessing quality healthcare due to geographical isolation, lack of transportation, and socioeconomic factors.
- Stigma and Discrimination further hinder access to healthcare services.
What are the Government Initiatives Regarding SCD?
- National Sickle Cell Anaemia Elimination Mission:
- Aimed at enhancing the care for all Sickle Cell Disease (SCD) patients and reducing the disease's prevalence through an integrated approach encompassing screening and awareness campaigns.
- Targeting complete elimination of sickle cell disease as a public health concern by 2047.
- Under the Sickle Cell Anaemia Mission, the Council of Scientific and Industrial Research (CSIR) is developing gene-editing therapies for SCD.
- National Health Mission (NHM) 2013:
- It is, a flagship programme of the Indian government, that encompasses provisions for disease prevention and management, with a specific focus on hereditary anomalies such as sickle cell anaemia.
- Dedicated programs within NHM focus on raising awareness, facilitating early detection, and ensuring timely treatment of sickle cell anaemia.
- NHM facilitates drugs like hydroxyurea to treat SCD in its “essential medicines List”.
- The National Guidelines for Stem Cell Research 2017:
- It restricts the commercialisation of stem cell therapies to clinical trials, except for Bone marrow transplantation (BMT) for SCD.
- Gene editing on stem cells is permitted only for in-vitro studies.
- National Guidelines for Gene Therapy Product Development and Clinical Trials 2019: It provides guidelines for the development and clinical trials of gene therapies for inherited genetic disorders.
- India has also approved a five-year project to develop CRISPR techniques for sickle cell anaemia treatment.
- State Haemoglobinopathy Mission of Madhya Pradesh:
- It aims to address the challenges in screening and management of the disease.
- Rights of Persons with Disabilities (RPwDs) Act, 2016:
- SCD is included in the 21 disabilities that provide for benefits such as reservation in higher education (minimum 5%), government jobs (minimum 4%), and allocation of land (minimum 5%), for persons with benchmark disabilities and those with high support needs.
- Free education is guaranteed for every child with a benchmark disability between 6 and 18 years.
Note
- Recently, the US Food and Drug Administration (FDA) approved two gene therapies designed for sickle cell disease.
- The approved therapies include Lyfgenia and Casgevy.
- Both treatments received clearance for individuals aged 12 years and above.
- Casgevy also approved in the U.K., is the first CRISPR-based therapy to have received regulatory approval.
- Lyfgenia doesn’t use CRISPR but depends on a viral vector to change blood stem cells.
- Both treatments entail collecting a patient’s blood stem cells, modifying them, and administering high-dose chemotherapy to destroy the damaged cells in the bone marrow.
- The modified cells are then infused into the patient through a hematopoietic stem cell transplant.
Way Forward
- Early Detection and Screening:
- Strengthen and expand genetic counselling and testing programmes.
- Prioritising basic treatments like hydroxyurea is essential for urgent needs.
- Identify carriers at an early stage to provide essential information to affected families.
- Ensuring equitable access to clinical trials is important to address the issue from the grassroots level.
- Public Education and Awareness:
- Implement sustained public awareness initiatives.
- Educate communities about the disease's hereditary nature and the importance of genetic testing.
- Public involvement in regulatory discussions is necessary.
- Research and Development:
- Allocate resources for ongoing research.
- Gain deeper insights into the genetic and molecular aspects of SCD to develop more effective treatment options and potential cures.
- Comprehensive healthcare access is vital for better long-term health outcomes.
UPSC Civil Services Examination Previous Year Question (PYQ)
Prelims:
Q. Consider the following statements in the context interventions being undertaken under Anemia Mukt Bharat Strategy : (2023)
- It provides prophylactic calcium supplementation for pre-school children, adolescents and pregnant women.
- It runs a campaign for delayed cord clamping at the time of child-birth.
- It provides for periodic deworming to children and adolescents.
- It addresses non-nutritional causes of anaemia in endemic pockets with special focus on malaria, hemoglobinopathies and fluorosis.
How many of the statements given above are correct?
(a) Only one
(b) Only two
(c) Only three
(d) All four
Ans: (c)
Mains:
Q. What are the research and developmental achievements in applied biotechnology? How will these achievements help to uplift the poorer sections of society? (2021)