Selenium-Graphene based Catalyst in Fuel Cell | 17 Jun 2019
A multi-institutional team of scientists from India has developed a selenium-graphene–based catalyst which is more efficient in terms of cost and performance. It also remains stable for longer than the usual platinum-based catalysts.
- Modern energy technology, for example, fuel cells which are used commercially in hydrogen fuel–based cars, require good catalysts that are efficient as well as cost-effective.
- Normally, fuel cells use expensive elements like platinum. These expensive metal-based technologies perform excellently for initial few cycles, but then get degraded in performance due to many reasons:
- Graphene modified with selenium atoms in very low amounts can perform like platinum in a demonstrated reaction.
- The oxygen reduction reaction is a key step in the functioning of the fuel cell. Graphene by itself is a “poor” catalyst of this reaction. It involves the reduction of oxygen in two steps, each of which consumes two electrons. This is not very useful either for fuel cells or metal-air batteries.
- Neither selenium nor graphene are useful by themselves, the combination of both works efficiently.
- Poisoning-resistant: Methanol fuel cells, a common form of fuel cell used, suffer from a “poisoning” effect. It is found that the catalyst that is developed has a high tolerance for poisoning.
Poisoning effect
- This is a part of the process where the methanol reaches the negative electrode and coats it, the electrode becomes ineffective after some cycles. This is especially problematic when expensive catalysts like platinum are used.
Selenium
- Selenium is a non-metallic chemical element, member of the group XVI of the periodic table. In chemical activity and physical properties, it resembles sulfur and tellurium.
- Selenium has good photovoltaic and photoconductive properties, and it is used extensively in electronics, such as photocells, light meters and solar cells.
Graphene: it is a one-atom-thick layer of carbon atoms arranged in a hexagonal lattice. It is the building-block of Graphite (which is used, among other things, in pencil tips).
- Graphene is the thinnest material known to man at one atom thick, and also incredibly strong - about 200 times stronger than steel.
- It is an excellent conductor of heat and electricity and has interesting light absorption abilities.
Fuel cell
- Fuel cells are electrochemical devices that convert chemical energy from the reactants directly into electricity and heat.
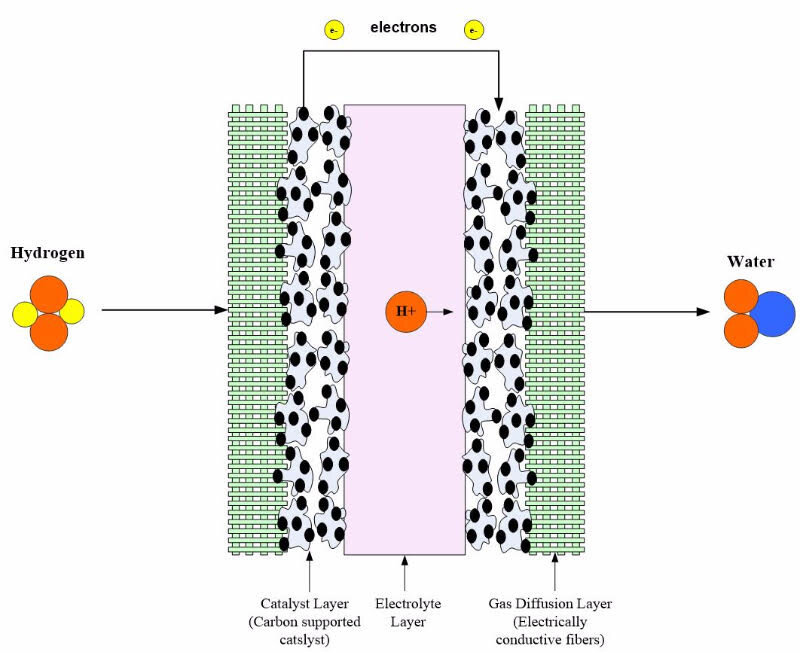
- The device consists of an electrolyte layer in contact with a porous anode and cathode on either side.
- In a standard fuel cell, gaseous fuels are fed continuously to the anode (negative electrode), while an oxidant (oxygen from the air) is fed continuously to the cathode (positive electrode). Electrochemical reactions take place at the electrodes to produce an electric current.
- Advantages of fuel cell systems are:
- High operating efficiency is not a function of system size.
- A highly scalable design.
- Several types of potential fuel sources are available.
- Zero or near-zero greenhouse emissions.
- There are no moving parts in the fuel cell stack, which provides reliable, vibration-free operation.
- Nearly instantaneous recharge capability when compared to batteries.
- Limitations of fuel cell systems are:
- Cost-effective, mass-produced pure hydrogen storage and delivery technology.
- Fuel Reformation technology may need to be considered if pure fuel is not used.
- Fuel cell performance may gradually decrease over time due to catalyst degradation and electrolyte poisoning if pure fuel is not used.
- Comparison of the fuel cell with Battery
- The lifetime of a primary battery is limited due to the following:
- The battery stops producing electricity when the chemical reactants stored in a battery runs out.
- When a battery is not being used, a very slow electrochemical reaction takes place that limits the lifetime of the battery.
- The battery life is dependent on the lifetime of the electrode.
- Advantage: A fuel cell is an energy conversion device where the reactants are supplied. The fuels are stored outside the fuel cell. A fuel cell can supply electrical energy as long as fuel and oxidant are supplied. Also, no “leakage” occurs in a fuel cell, and no corrosion of cell components occurs when the system is not in use.
- The lifetime of a primary battery is limited due to the following:
- Comparison of the fuel cell with Heat Engine
- A heat engine also converts chemical energy into electric energy, but through intermediate steps. The chemical energy is first converted into thermal energy through combustion. Thermal energy is then converted into mechanical energy by the heat engine; and Finally, the mechanical energy is converted into electric energy by an electric generator.
- The process also involves moving parts, which implies that they wear over time. Regular maintenance of moving components is required for proper operation of the mechanical components.
- Advantage: Since fuel cells do not have any moving parts during operation, they are more reliable than heat engines and have less noise. This results in lower maintenance costs, which make them especially advantageous for space and underwater missions.
- The efficiency of fuel cells is not strongly dependent on operating power. It is their inherently high efficiency that makes fuel cells an excellent option for a broad range of applications, including automobiles, buses, distributed electricity, and portable systems.
