Science & Technology
Nanobodies
- 14 Jan 2021
- 5 min read
Why in News
An international research team led by the University of Bonn (Germany) has identified and further developed novel antibody fragments (nanobodies) against SARS-CoV-2, the virus that causes Covid-19.
Key Points
- Nanobodies Against SARS-CoV-2:
- Produced along with Antibodies: On injection of surface protein of the coronavirus into an alpaca and a llama, their immune system not only produced antibodies directed against the virus but also a simpler antibody variant that can serve as the basis of nanobodies.
- More Effective:
- They had also combined the nanobodies into potentially particularly effective molecules, which attack different parts of the virus simultaneously. This new approach could prevent the pathogen from evading the effect of antibodies through mutations.
- Nanobodies appear to trigger a structural change before the virus encounters its target cell - an unexpected and novel mode of action. The change is likely to be irreversible; the virus is therefore no longer able to bind to host cells and infect them.
- Antibodies:
- Antibodies are an important weapon in the immune system’s defense against infections.
- They bind to the surface structures of bacteria or viruses and prevent their replication.
- One strategy in the fight against disease is therefore to produce effective antibodies in large quantities and inject them into patients. However, producing antibodies is difficult and time-consuming; they are, therefore, probably not suitable for widespread use.
- Nanobodies:
- Nanobodies are antibody fragments that are so simple that they can be produced by bacteria or yeast, which is less expensive.
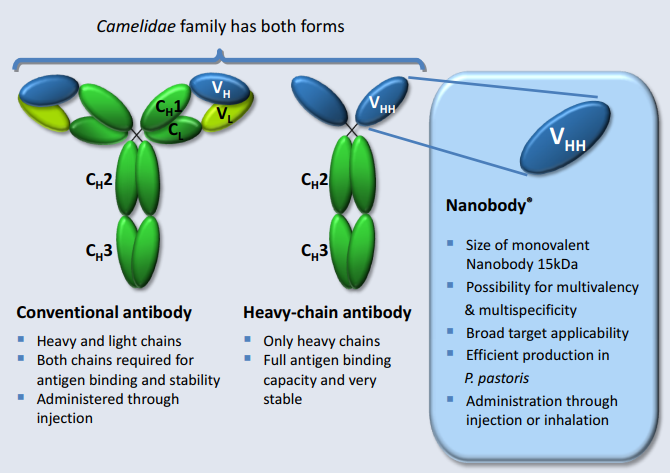
- These are antibodies with a single variable domain located on a heavy chain, also known as VHH antibodies.
- These are often seen as an alternative to conventional antibodies, and have significant differences in both production and use that influence their suitability.
- Difference between Nanobodies and Conventional Antibodies:
- Difference in Structure and Domains:
- Conventional antibodies have two variable domains, called VH and VL, which offer each other stability and binding specificity.
- Nanobodies have VHH domains and lack VL domains, but are still highly stable. Lacking the VL domain also means nanobodies have a hydrophilic (having a tendency to dissolve in a water) side.
- Hydrophilic side means they do not have issues with solubility and aggregation otherwise associated with conventional antibodies.
- Nanobody production follows many of the same protocols as used in traditional antibody production. However, it also has distinct advantages not available with traditional antibodies, such as improved screening, improved isolation techniques, and no animal sacrifice.
- Difference in Structure and Domains:
- Use:
- Nanobodies are much smaller than classic antibodies and they, therefore, penetrate the tissue better and can be produced more easily in larger quantities.
- Nanobodies are stable in a wide range of temperatures, remaining functional at temperatures as high as 80°C. As an added bonus, unfolding of the nanobody due to high temperatures has been shown to be fully reversible, unlike conventional antibody fragments.
- Nanobodies are also stable at extreme pH levels, able to survive exposure to gastric fluid.
- Nanobodies are also compatible with genetic engineering methods, which allow alteration of amino acids to improve binding.
- Limitations of Nanobodies:
- Monoclonal and polyclonal antibodies are slightly safer to produce than nanobodies, as there are biohazards involved in nanobody production not present for conventional antibody production.
- The biohazards result mainly from use of hazardous bacteriophages (any of a group of viruses that infect bacteria) for selection of nanobodies. Other sources include plasmids, antibiotics, and recombinant DNA. These materials require safe disposal.
- Polyclonal antibodies are made using several different immune cells.
- Monoclonal antibodies are made using identical immune cells that are all clones of a specific parent cell.
- The biohazards result mainly from use of hazardous bacteriophages (any of a group of viruses that infect bacteria) for selection of nanobodies. Other sources include plasmids, antibiotics, and recombinant DNA. These materials require safe disposal.
- Monoclonal and polyclonal antibodies are slightly safer to produce than nanobodies, as there are biohazards involved in nanobody production not present for conventional antibody production.