Important Facts For Prelims
Allotropes of Carbon
- 21 Nov 2024
- 5 min read
Why in News?
Carbon and its allotropes remain in news due to its varied physical and chemical properties.
- Allotrope refers to one or more forms of a chemical element that occur in the same physical state.
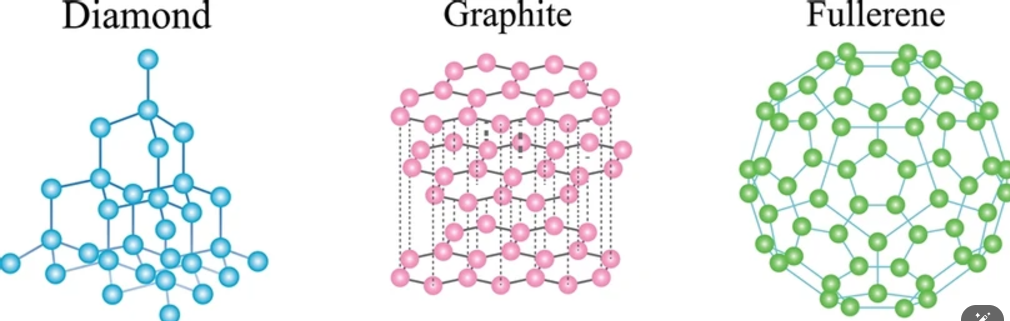
- Carbon has four main allotropes namely Diamond, Graphite, Fullerenes, and Graphene.
- Additionally, carbon nanotubes and amorphous carbon (like charcoal) are also considered forms of carbon, but they are less commonly classified as primary allotropes.
What are the Allotropes of Carbon?
- Graphite: In graphite, each carbon atom forms bonds with three other carbon atoms, creating two-dimensional sheets. It is made up of layers of Carbon atoms arranged in hexagonal arrays.
- Electricity Conduction: Graphite is a good conductor of electricity due to the existence of delocalised electrons within its layers.
- Lubricant: Its layers can easily slide over each other, making it suitable as a solid lubricant.
- Hardness: Graphite is the softest carbon allotrope.
- Graphene: Graphene is a single, one atom thick layer of graphite. It has vast potential in electronics, energy storage, sensors, coatings, composites, and biomedical devices.
- Its high surface area and biocompatibility make it ideal for drug delivery and tissue engineering.
- Diamond: It is made up of a three-dimensional network of Carbon atoms arranged in a tetrahedral structure, where each carbon atom is bonded to other four carbon atoms.
- Hardness: It is recognized as the hardest naturally occurring material due to its strong covalent bonds, making it suitable for industrial cutting, drilling, and grinding applications.
- Transparency: Some diamonds exhibit high transparency in the visible spectrum, making them valuable in jewellery.
- Thermal Conductivity: Diamonds possess excellent thermal conductivity, making them useful in heat sinks.
- Electricity Conduction: It lacks electrical conductivity in its pure form as it has no free electrons or "charge carriers" available to conduct electricity.
- Lab-grown Diamonds (LGDs): LGDs are identical to natural diamonds in terms of physical properties such as hardness, sparkle, and durability but are created artificially in laboratories using Graphite as a diamond seed.
- Fullerene: Buckminsterfullerene is a type of fullerene with the formula C60 and is characterised by its distinctive cage-like structure resembling a football.
- Applications: Fullerenes and their compounds have potential applications as semiconductors, superconductors, lubricants, catalysts, electric wires, and plastic reinforcing fibres.
- Carbon Nanotubes: They are cylindrical structures made of rolled-up graphene sheets.
- They are used in electronics, materials science, energy storage, medical applications, sensors, water purification, drug delivery, aerospace, and nanotechnology.
- They can be used as carriers of drugs and antigens in the human body and biochemical sensors.
- They are biodegradable in nature.
- Amorphous Carbon: It refers to various forms of carbon lacking a crystalline structure, such as charcoal, soot, and activated carbon.
UPSC Civil Services Examination, Previous Year Questions (PYQs)
Prelims
Q.With reference to carbon nanotubes, consider the following statements: (2020)
- They can be used as carriers of drugs and antigens in the human body.
- They can be made into artificial blood capillaries for an injured part of human body.
- They can be used in biochemical sensors.
- Carbon nanotubes are biodegradable.
Which of the statements given above are correct?
(a) 1 and 2 only
(b) 2, 3 and 4 only
(c) 1, 3 and 4 only
(d) 1, 2, 3 and 4
Ans: (c)
Q. Graphene is frequently in news recently. What is its importance? (2012)
- It is a two-dimensional material and has good electrical conductivity.
- It is one of the thinnest but strongest materials tested so far.
- It is entirely made of silicon and has high optical transparency
- It can be used as ‘conducting electrodes’ required for touch screens, LCDs and organic LEDs.
Which of the statements given above are correct?
(a) 1 and 2 only
(b) 3 and 4 only
(c) 1, 2 and 4 only
(d) 1, 2, 3 and 4
Ans: (c)