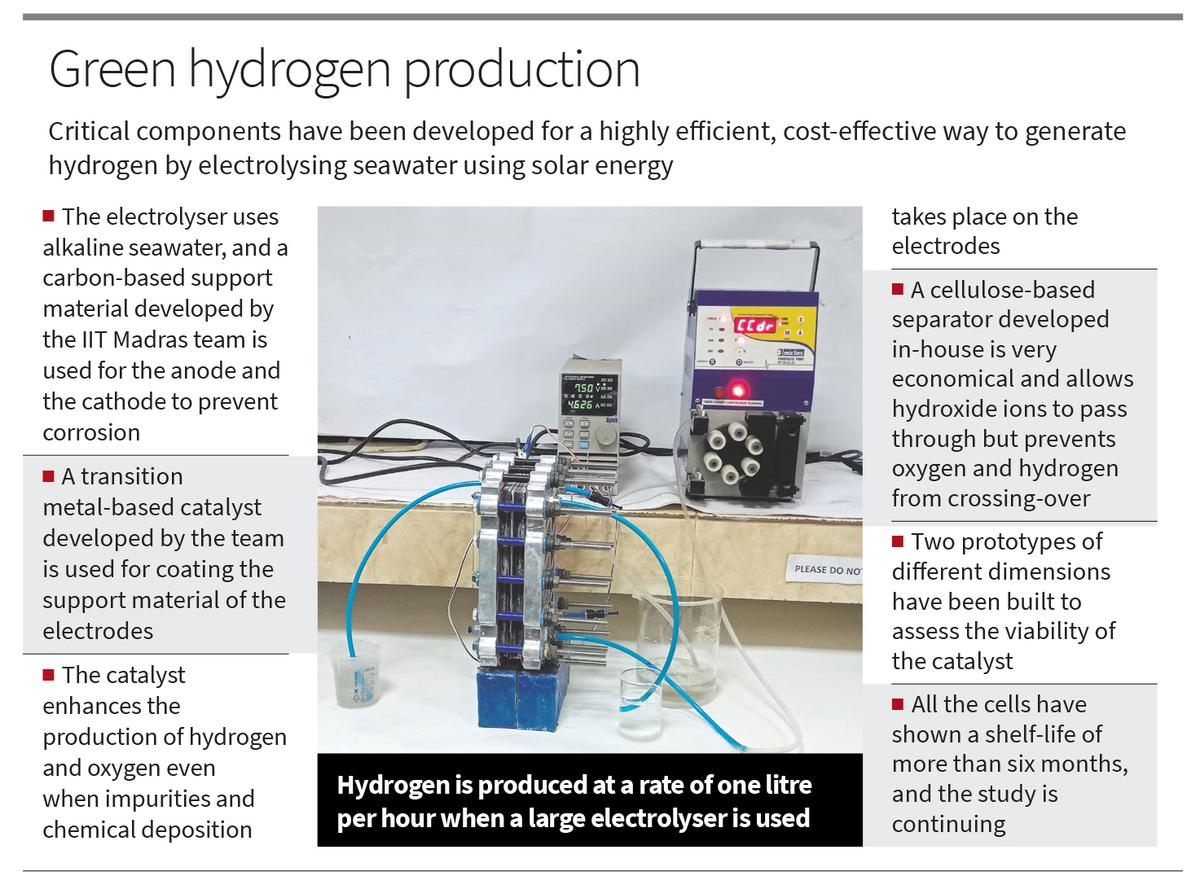
Alkaline Seawater Electrolyzer for Hydrogen Generation | 13 Jun 2023
Why in News?
Recently, Researchers from IIT-Madras have developed Alkaline Seawater Electrolyzer to Generate Hydrogen, addressing the challenges related with the existing Water Electrolyzer Technology.
- Alkaline water electrolyzer technology is energy-intensive, requires an expensive oxide-polymer separator, and uses fresh water for electrolysis. This invention has addressed each of these challenges by developing simple, scalable and cost-effective alternatives that are highly efficient in splitting seawater and generating hydrogen.
What are the Key Features of the Invention?
- Carbon Based Support Catalyst:
- In an Alkaline Water electrolyzer, two reactions occur at the anode and cathode. At the cathode, water splits into H+ and hydroxide ions. The H+ ions become hydrogen, while the hydroxide ions pass through a separator and form oxygen at the anode.
- However, when seawater is used, there are challenges. The anode forms hypochlorite, which corrodes the electrode support material and competes with oxygen production. The cathode gets impurities that slow down hydrogen production.
- To solve these issues, the electrodes have a special support material coated with a catalyst. Instead of using metals that corrode seawater, a carbon-based material is used.
- This support material, found in both the anode and cathode, is coated with the catalyst. The catalyst enables improved production of hydrogen and oxygen simultaneously.
- Cellulose-Based Separator:
- Usually, an expensive zirconium oxide-based material is used to separate the anode and cathode in alkaline electrolysis.
- However, the researchers have used a Cellulose-Based Separator. This separator allows hydroxide ions to pass from the cathode to the anode while minimizing the crossover of hydrogen and oxygen.
- This separator is highly resistant to degradation when exposed to seawater. This is an important quality for long-term performance and durability.
What is the Significance of this Invention?
- This invention addresses the limitations of current technologies and paves the way for scalable and sustainable hydrogen production, contributing to a greener and more sustainable future.
What are the Reasons to Develop Green Hydrogen?
- Reducing Greenhouse Gas Emissions:
- The main goal of green hydrogen is to reduce harmful greenhouse gas emissions, which are a major cause of climate change.
- Unlike traditional fuels, green hydrogen produced from renewable sources emits no greenhouse gases, making it an eco-friendly and sustainable energy option.
- Energy Security and Independence:
- By shifting towards renewable energy sources like green hydrogen, countries can become more self-reliant and less vulnerable to price fluctuations and supply disruptions associated with finite fossil fuels. This enhances energy security and independence.
- Decarbonizing Difficult-to-Decarbonize Sectors:
- Green hydrogen has great potential to replace fossil fuels in sectors that are challenging to decarbonize, such as heavy industry and aviation. These sectors contribute significantly to global emissions, and adopting green hydrogen can help reduce their carbon footprint.
- Technological Advancements:
- The advancement of green hydrogen technology drives innovation in various fields. Developing the infrastructure for production, storage, and distribution of green hydrogen requires new technologies, materials, and systems. This stimulates progress and breakthroughs in related industries.
UPSC Civil Services Examination, Previous Year Questions (PYQ)
Q1. Consider the following heavy industries: (2023)
- Fertilizer plants
- Oil refineries
- Steel plants
Green hydrogen is expected to play a significant role in decarbonizing how many of the above industries?
(a) Only one
(b) Only two
(c) All three
(d) None
Ans: (c)
Q2. With reference to green hydrogen, consider the following statements: (2023)
- It can be used directly as a fuel for internal combustion.
- It can be blended with natural gas and used as fuel for heat or power generation.
- It can be used in the hydrogen fuel cell to run vehicles.
How many of the above statements are correct?
(a) Only one
(b) Only two
(c) All three
(d) None
Ans: (c)