Science & Technology
150 years of the Periodic Table
- 11 Feb 2019
- 4 min read
The United Nations has designated 2019 as the International Year of the Periodic Table (IYPT2019) to commemorate the 150th anniversary of the establishment of the Periodic Table of Chemical Elements by Dmitri Ivanovich Mendeleev on 17th February 1869.
- The International Year aims to recognize the importance of the Periodic Table of Chemical Elements as one of the most important and influential achievements in modern science reflecting the essence not only of chemistry but also of physics, biology and other basic sciences disciplines.
- The initiative for IYPT2019 is supported by the International Union of Pure Applied Chemistry (IUPAC) in partnership with other science-related organization.
- Mendeleev was not the first one to create a table of elements. Earliest of such efforts was due to the father of modern chemistry, Antoine Lavoisier in 1789 who classified them in terms of their properties.
- John Newlands introduced the concept of octaves in chemistry, wherein properties repeat for every eighth element.
- In comparison to the modern periodic table, Mendeleev's periodic table was designed in the order of increasing atomic weight while the modern periodic table is designed according to increasing atomic number.
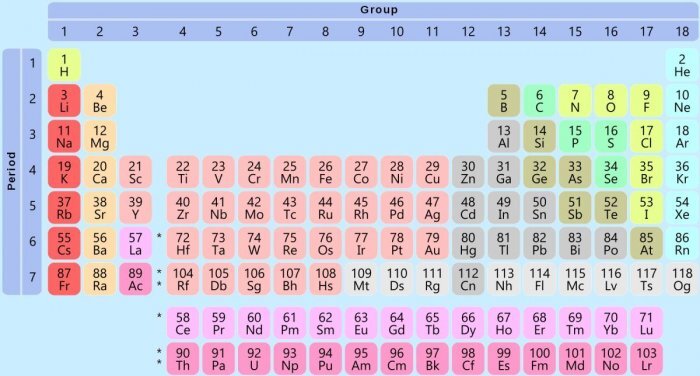
Important Facts about Periodic Table
- There are 118 confirmed elements in the periodic table. Among those, 90 elements can be found in nature, others are strictly man-made. Technetium was the first man-made element.
- Hydrogen is the lightest element with its atomic weight 1. Uranium is the heaviest element with an atomic weight of 238.
- Helium, neon, argon, krypton, xenon, and radon are known as the Noble Gases as they were believed to be unreactive. But recent studies have shown reactive compounds of xenon, krypton, and radon.
- The IUPAC is responsible for maintaining the periodic table.
- Most of the elements on the periodic table are metals (almost 75 percent).
- Different forms of pure elements are called allotropes. For example, diamond, graphite, buckminsterfullerene, and amorphous carbon are allotropes of Carbon.
- The only two elements that are liquid in room temperature are mercury and bromine.
The International Union of Pure and Applied Chemistry (IUPAC)
- IUPAC is the world authority on chemical nomenclature and terminology, including the naming of new elements in the periodic table; on standardized methods for measurement; and on atomic weights etc.
- A neutral and objective scientific organization, IUPAC was established in 1919 by academic and industrial chemists who shared a common goal – to unite a fragmented, global chemistry community for the advancement of the chemical sciences via collaboration and the free exchange of scientific information.
- Four new elements discovered in 2015 have been named by the International Union of Pure and Applied Chemistry (IUPAC).
- These are Nihonium (113Nh), Moscovium (115Mo), Tennessine (117Te) and Oganesson (118Og).
- Of these elements, Nh-278 is highly radioactive with a very short half-life of 0.24 milliseconds.