Biodiversity & Environment
Imbalance in Nitrogen Availability
- 20 Apr 2022
- 6 min read
For Prelims: Nitrogen, light pollution, invasive species, wildfire, carbon dioxide
For Mains: Importance of Nitrogen Cycle
Why in News?
According to a new report, an imbalance in nitrogen availability has been reported across the globe, with some places having an excess and others a shortage of the element.
What are the Reasons Causing the Decline?
- Rising carbon dioxide levels and other global changes have increased demand for nitrogen by plants and microbes.
- Plants grow quickly when exposed to high carbon dioxide (CO2) concentrations.
- The presence of high CO2 levels dilutes the availability of nitrogen in Plants, thus, their demand for nitrogen goes up.
- Other factors contributing to nitrogen decline include warming and disturbances, including wildfire.
- Many areas of the world, where people do not contribute excessive amounts of nitrogen to the soil, long-term records demonstrate that nitrogen availability is declining, with important consequences for plant and animal growth.
- Burning fossil fuels, application of nitrogen-based fertilizers, and other activities can dramatically increase the amount of biologically available nitrogen in an ecosystem.
What are the Consequences of Nitrogen Imbalance?
- Low Nitrogen:
- Declining nitrogen availability can be linked to insect apocalypse.
- Climate change, insecticides, herbicides, light pollution, invasive species and changes in agriculture and land use are causing Earth to lose about 1-2% of its insects each year. This is being termed as “Insect Apocalypse”.
- It can encourage swarming in some species of locusts.
- Further, low nitrogen availability could limit plants’ ability to capture CO2 from the atmosphere.
- Declining nitrogen availability can be linked to insect apocalypse.
- High Nitrogen:
- When excessive nitrogen accumulates in the streams, inland lakes and coastal bodies of water, it could sometimes result in eutrophication, leading to harmful algal blooms, dead zones and fish kills.
- Eutrophication: When a water body becomes overly enriched with minerals and nutrients which induce excessive growth of algae or algal bloom. This process also results in oxygen depletion of the water body.
- In humans, high levels of nitrogen in the groundwater are linked to intestinal cancers and miscarriages and can be fatal for infants.
- When excessive nitrogen accumulates in the streams, inland lakes and coastal bodies of water, it could sometimes result in eutrophication, leading to harmful algal blooms, dead zones and fish kills.
What are the Key Highlights about Nitrogen?
- Nitrogen is one of the primary nutrients critical for the survival of all living organisms.
- Nitrogen gas makes up 78% of our atmosphere and nitrogen is also a part of many molecules essential to life like proteins, nucleic acids (DNA and RNA) and some vitamins.
- Nitrogen is found in other biologically important compounds such as alkaloids and urea too.
- Nitrogen is thus an essential nutrient for all life-forms and life would be simple if all these life-forms could use the atmospheric nitrogen directly.
- Although nitrogen is abundant in the atmosphere as Nitrogen gas (N2), it is largely inaccessible in this form to most organisms, making nitrogen a scarce resource and often limiting primary productivity in many ecosystems.
- Only when nitrogen is converted from Nitrogen gas into ammonia (NH3) does it become available to primary producers, such as plants.
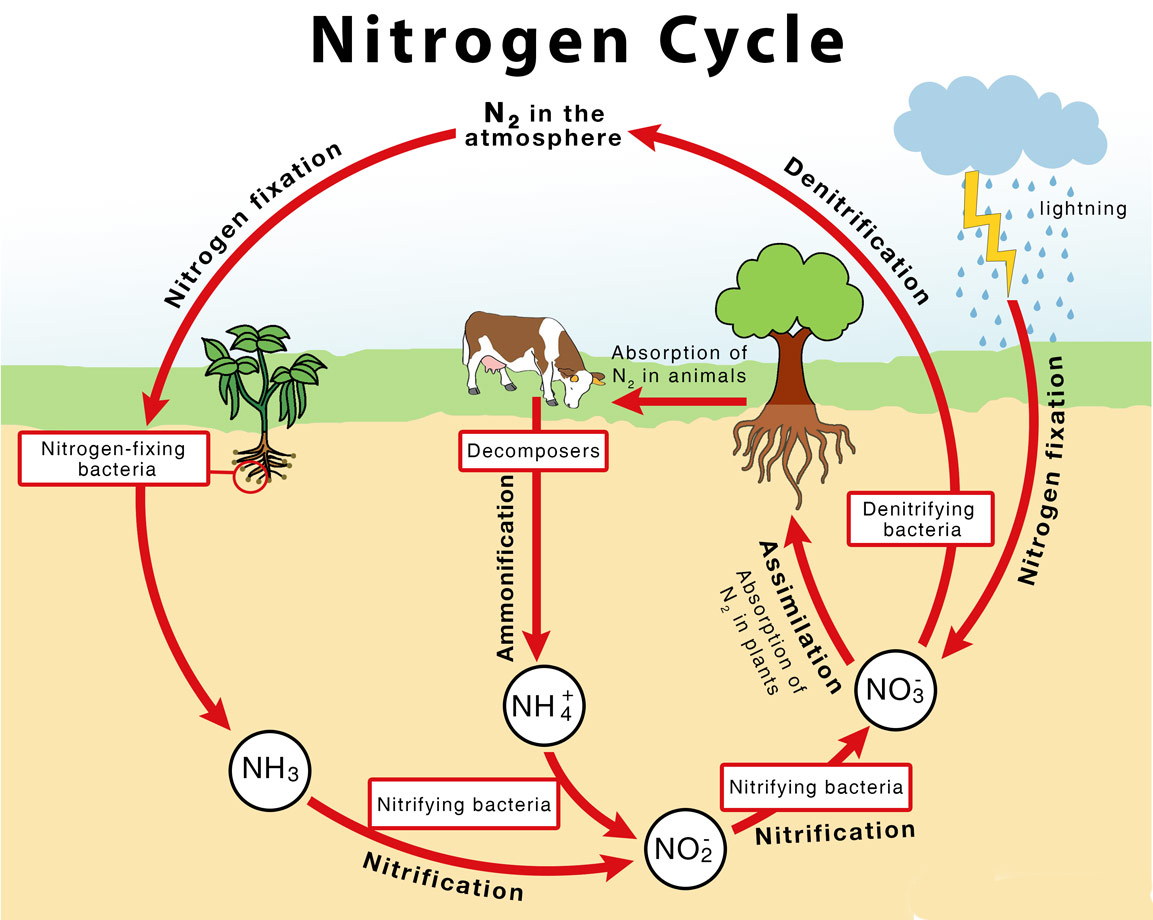
- The major transformations of nitrogen gas are through the process of:
- Nitrogen fixation (nitrogen gas to ammonia),
- Nitrification (ammonia to nitrite and nitrate),
- Denitrification (nitrate to nitrogen gases)
- The process of converting Nitrogen gas (N2) into biologically available nitrogen, that is ammonia, by nitrogen fixing microorganisms, is called nitrogen fixation.
- Some nitrogen-fixing organisms are free-living, while others are symbiotic nitrogen-fixers, which require a close association with the host to carry out the process.
- Some of these bacteria are aerobic, others are anaerobic; some are phototrophic, others are chemotrophic (use chemicals as their energy source instead of light).
- They all have a similar enzyme complex called nitrogenase that catalyzes the reduction of N2 to NH3 (ammonia).
Q. Which of the following adds/add nitrogen to the soil? (2013)
- Excretion of urea by animals
- Burning of coal by man
- Death of vegetation
Select the correct answer using the codes given below:
(a) 1 only
(b) 2 and 3 only
(c) 1 and 3 only
(d) 1, 2 and 3
Ans: (c)
- Mammals, including humans, are the primary producers of urea. Because they secrete urea as the primary nitrogenous waste product, they are called ureotelic animals. These wastes add nitrogen to the soil. Hence, 1 is correct.
- Coal combustion produces Oxides of Carbon (COx), Oxides of Sulphur (SOx), Oxides of Nitrogen (NOx) and a variety of byproducts, including fly-ash, flue gas and scrubber sludge. However, it does not directly add nitrogen to the soil. Hence, 2 is not correct.
- When plants and animals die, the Nitrogen compounds in the organic matter re-enter the soil where they are broken down by microorganisms, known as decomposers. This decomposition produces Ammonia, which goes through the nitrification process, i.e., nitrifying bacteria in the soil convert Ammonia into Nitrite (NO2 ) and then into Nitrate (NO3 ). Hence, 3 is correct. Therefore, option (c) is the correct answer.