Converting CO2 to Methane | 28 Oct 2021
Why in News
Recently, Indian Scientists have designed a photochemical method (Photocatalyst) to convert Carbon Dioxide (CO2)to Methane (CH4).
- A photochemical method is a chemical reaction initiated by the absorption of energy in the form of light.
Key Points
- About:
- A polymer has been designed to absorb visible light and catalyse the reaction which reduces CO2.
- Most catalysts contain toxic and expensive metal counterparts. Therefore scientists designed a metal-free porous organic polymer to overcome this drawback.
- The photochemical method of reducing CO2 uses solar light as a renewable source of energy.
- There are several ways in which CO2 can be reduced, including photochemical, electrochemical, photoelectrochemical, photothermal, and so on.
- A polymer has been designed to absorb visible light and catalyse the reaction which reduces CO2.
- Mechanism:
- The catalyst has a chemical called the Conjugated Microporous Polymer (CMP).
- It can uptake CO2 onto its surface due to its high CO2 intake capability at room temperature, converting it into methane as a value-added product.
- There are some key requirements of a photo-catalyst to convert CO2 into value-added products, which rely upon:
- Light-harvesting property.
- Charge carrier (electron-hole pair) separation proficiency.
- Presence of proper electronically aligned conduction band.
- Significance:
- Methane can be one of the value-added products with significant uses as the cleanest burning fossil fuel and can directly be used in fuel cells as a hydrogen carrier.
- It is also the main component of natural gas and has the potential to replace coal for electricity generation and furnishing flexible supply to reinforce intermittent renewable generators.
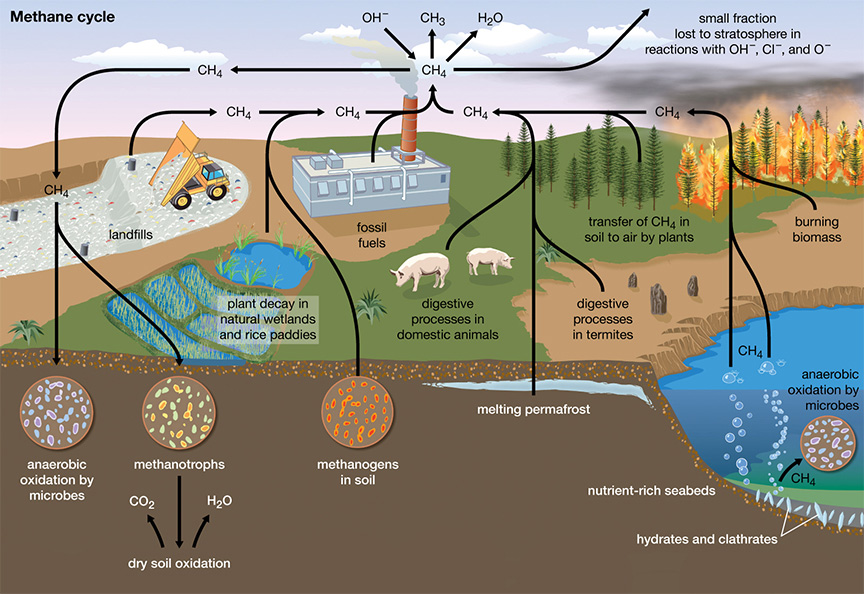
Methane
- About:
- Methane is gas that is found in small quantities in Earth's atmosphere.
- It is the simplest hydrocarbon, consisting of one carbon atom and four hydrogen atoms (CH4).
- Methane is a powerful greenhouse gas. It is flammable, and is used as a fuel worldwide.
- Methane is produced by the breakdown or decay of organic material and can be introduced into the atmosphere by either natural processes – such as the decay of plant material in wetlands, the seepage of gas from underground deposits or the digestion of food by cattle – or human activities – such as oil and gas production, rice farming or waste management.
- Methane is called marsh gas because it is found at the surface of marshy places
- Major Uses:
- It is an important source of hydrogen and some organic chemicals.
- It reacts with steam at high temperatures to yield carbon monoxide and hydrogen; the latter is used in the manufacture of ammonia for fertilizers and explosives.
- Other valuable chemicals derived from methane include methanol, chloroform, carbon tetrachloride, and nitromethane.
- The incomplete combustion of methane yields carbon black, which is widely used as a reinforcing agent in rubber used for automobile tires.
- Environmental Impact of Methane:
- It is 84 times more potent than carbon and doesn’t last as long in the atmosphere before it breaks down. This makes it a critical target for reducing global warming more quickly while simultaneously working to reduce other greenhouse gases.
- It is responsible for creating ground-level ozone, a dangerous air pollutant.